Advertisements
Advertisements
Question
[Ni(CO)4] has tetrahedral geometry while [Ni(CN)4]2− has square planar, yet both exhibit diamagnetism. Explain.
[Atomic number: Ni = 28]
Solution
In [Ni(CN)4]2−, nickel is in a +2 oxidation state and the ion has the electronic configuration 3d8. The hybridisation scheme is shown in the diagram.
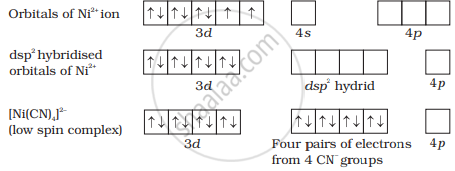
Whereas in [Ni(CO)4], Ni is in a +2 oxidation state and shows sp2 hybridisation due to which its geometry is tetrahedral.
APPEARS IN
RELATED QUESTIONS
[NiCl4]2− is paramagnetic, while [Ni(CO)4] is diamagnetic, though both are tetrahedral. Why? (Atomic number of Ni = 28)
Explain on the basis of valence bond theory that [Ni(CN)4]2− ion with square planar structure is diamagnetic and the [NiCl4]2− ion with tetrahedral geometry is paramagnetic.
Discuss the nature of bonding in the following coordination entity on the basis of valence bond theory:
[FeF6]3−
Explain the geometry of `[Co(NH_3)_6]^(3+)` on the basis of hybridisation. (Z of Co = 27)
Using valence bond theory, explain the following in relation to the complexes given below:
\[\ce{[Mn(CN)6]^{3-}}\]
(i) Type of hybridisation.
(ii) Inner or outer orbital complex.
(iii) Magnetic behaviour.
(iv) Spin only magnetic moment value.
Which of the statement given below is incorrect about H2O2?
As the s-character of hybridised orbital increases, the bond angle
Which of the following methods is used for measuring bond length?
Which of the following has square planar structures?
During chemistry class, a teacher wrote \[\ce{[Ni(CN)4]^2-}\] as a coordination complex ion on the board. The students were asked to find out the magnetic behaviour and shape of the complex. Pari, a student, wrote the answer paramagnetic and tetrahedral whereas another student Suhail wrote diamagnetic and square planer.
Evaluate Pari’s and Suhail’s responses.
