Advertisements
Advertisements
प्रश्न
In an isochoric process, we have ____________.
पर्याय
W = 0
Q = 0
∆U = 0
∆T = 0
उत्तर
In an isochoric process, we have W = 0.
APPEARS IN
संबंधित प्रश्न
An ideal gas is taken through an isothermal process. If it does 2000 J of work on its environment, how much heat is added to it?
Draw a p-V diagram showing positive work with varying pressure.
Explain work done during a thermodynamic process.
Explain the thermodynamics of the isochoric process.
When food is cooked in a vessel by keeping the lid closed, after some time the steam pushes the lid outward. By considering the steam as a thermodynamic system, then in the cooking process
Draw the PV diagram for the isothermal process.
Draw the PV diagram for the isobaric process.
A monoatomic gas of pressure p having volume V expands isothermally to a volume 2V and then adiabatically to a volume 16V. The final pressure of the gas is ____________.
`("ratio of specific heats" = 5/3)`
An ideal gas is taken through a cyclic process ABCDA as shown in figure. The net work done by the gas during the cycle is ______.
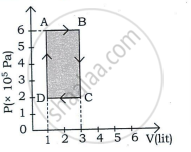
In a cyclic process, if ΔU = internal energy, W = work done, Q = Heat supplied then ______.
