Advertisements
Advertisements
प्रश्न
In Period 3 of the Periodic Table, element B is placed to the left of element A. On the basis of this information, choose the correct word from the option to complete the following statement:
The element A would have ______ atomic size than B.
पर्याय
greater
smaller
उत्तर
The element A would have smaller atomic size than B.
APPEARS IN
संबंधित प्रश्न
Which is greater in size an atom or a cation?
Size of atom progressively becomes smaller when we move from sodium (Na) to chlorine (CI) in the third period of the periodic table?
Fill in the blank:
On moving across a period from right to left in the periodic table, the atomic size of the atom ___________.
Write the name and symbol of the element from the description.
The noble gas with the smallest atomic radius.
What happens to the atomic size of elements on moving from left to right in a period?
Write scientific reason.
In same period, boron and oxygen elements have different atomic size.
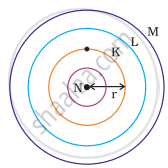
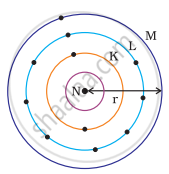
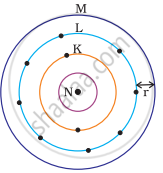
Which one of the following depict the correct representation of atomic radius(r) of an atom?
|
(i) |
(ii) |
|
(iii) |
(iv) |
Arrange the following in order of increasing radii:
Mg2+, Mg, Mg+
Explain your choice.
Arrange the following in order of increasing radii:
N, O, P
Explain your choice.
Give reason for the following:
The size of a Cl− ion is greater than the size of a Cl atom.