Advertisements
Advertisements
प्रश्न
Arrange the following in order of increasing radii:
N, O, P
Explain your choice.
उत्तर
O < N < P
Explanation:
N(7) = 2, 5; O(8) = 2, 6; P(15) = 2, 8, 5
Radius of oxygen < Radius of Nitrogen where as phosphorus has three shells.
संबंधित प्रश्न
Answer the following in respect of element `31/15 P`
Give its electronic configuration
Give the trends in atomic size on moving across the period left to right.
Give reason for the following:
Argon atom is bigger than chlorine atom.
Write the name and symbol of the element from the description.
The atom having the smallest size.
Arrange the following as per the instruction given in the bracket.
Mg, Cl, Na, S, Si (increasing order of atomic size)
The size of an atom is indicated by its _______.
Write an Explanation.
Atomic radius
Which among the following elements has the largest atomic radii?
The diagram below shows part of the periodic table.
- Which elements would react together to form covalent compounds?
- Between the two elements W and Z, which will have a bigger atomic radius? Why?
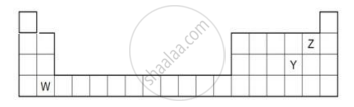
An element with the largest atomic radius among the following is ______.
