Advertisements
Advertisements
प्रश्न
Write an Explanation.
Atomic radius
उत्तर
For an isolated atom, the distance between the centre of the nucleus of the atom and the outermost shell is called its atomic radius.
APPEARS IN
संबंधित प्रश्न
Why is the size of sodium is greater than magnesium?
Give reason for the following:
Argon atom is bigger than chlorine atom.
Arrange the following in order of increasing radii:
CI- , CI
With reference to the variation of properties in the Periodic Table, which of the following is generally true?
Atomic size increases from left to right across a period.
Arrange the following as per the instruction given in the bracket.
Mg, Cl, Na, S, Si (increasing order of atomic size)
Arrange the following as per the instruction given in the bracket
Na, K, Li (Increasing atomic size)
The size of an atom is indicated by its _______.
Moving from left to right, the size of the atom decreases.
Write scientific reason.
In same period, boron and oxygen elements have different atomic size.
Some elements and their atomic radii are given here. Arrange them in decreasing order of their atomic radii. Identify which of the above elements is the biggest atom and which is smallest?
| Element | K | Na | Rb | Cs | Li |
| Atomic radius (pm) | 231 | 186 | 244 | 262 | 151 |
Carbon belongs to the second period and Group 14. Silicon belongs to the third period and Group 14. If the atomic number of carbon is 6, the atomic number of silicon is ______
Which of the following is the correct order of atomic size?
Which of the following gives the correct increasing order of the atomic radii of O, F and N?
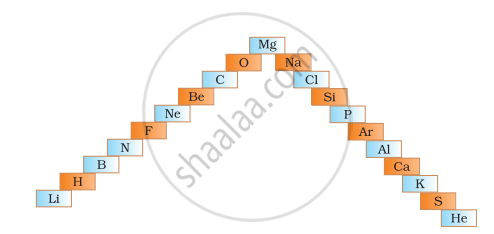
- In below ladder symbols of elements are jumbled up. Rearrange these symbols of elements in the increasing order of their atomic number in the Periodic Table.
- Arrange them in the order of their group also.
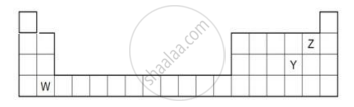
The diagram below shows part of the periodic table.
- Which elements would react together to form covalent compounds?
- Between the two elements W and Z, which will have a bigger atomic radius? Why?
Arrange the following as per the instruction given in the bracket:
Carbon, Fluorine, Beryllium (decreasing order of atomic size).
Give reason for the following:
The size of a Cl− ion is greater than the size of a Cl atom.
This question refers to the elements of the Periodic Table with atomic numbers from 3 to 18. Some of the elements are shown by letters, but the letters are not the usual symbols of the elements.
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| A | B | C | D | E | F | G | H |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| I | J | K | L | M | N | O | P |
Which of these have least atomic size in period 3?
