Advertisements
Advertisements
प्रश्न
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
Dilute nitric acid is not preferred as the reactant acid.
उत्तर
Hydrogen produced is oxidised to water as nitric acid is powerful oxidizing agent.
APPEARS IN
संबंधित प्रश्न
Indicate which of the following statement is true and which is false:
Zinc can liberate hydrogen from water, acid and alkali solution.
Draw a neat and well-labelled diagram for the laboratory preparation of hydrogen.
How is hydrogen gas collected? Why?
How would you show that hydrogen is a non-supporter of combustion?
Multiple Choice Question
In metal activity series the more reactive metals are at
FILL IN THE BLANK
Sodium liberates hydrogen when treated with cold .............
TRUE \ FALSE
Lead reacts briskly with dilute hydrochloric acid to form hydrogen.
Name the impurities present in hydrogen prepared in the laboratory.
How can these impurities be removed?
Under what conditions can hydrogen be made to combine with chlorine?
Name the products and write the equation for the reaction.
Starting from zinc how would you obtain hydrogen using Steam.
[Give balanced equation & name the product formed in the case other than hydrogen].
Name a metal which will not react with the reactants above to give hydrogen.
Draw neat labelled diagrams for two different experiments to prove that – hydrogen is lighter than air.
Give balanced equation for the following conversion:
Acidified water to hydrogen – by electrolysis.
Give a balanced equation for the following conversions sodium aluminate from aluminium.
In the laboratory preparation of hydrogen from zinc and dil. acid. Give a reason for the following:
Hydrogen is not collected over air.
Give a reason for the following.
Nitric acid in the dilute form is not used in the laboratory preparation of hydrogen from metals.
The diagram represent the preparation and collection of hydrogen by a standard
laboratory method.
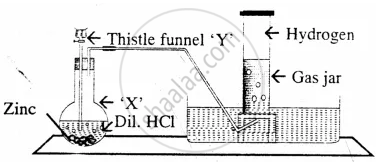
State what is added through the thistle funnel ‘Y’
The diagram represent the preparation and collection of hydrogen by a standard laboratory method.

Name a solution which absorbs the impurity – `"H"_2"S"`
