Advertisements
Advertisements
प्रश्न
Match the columns.
| Reactants | Products | Types of chemical reaction | ||
| 1. | MgH2 | → | Mg + H2 | Endothermic |
| 2. | 2H2S + SO2 | → | 3S + 2H2O | Oxidation |
| 3. | CaO + H2O | → | Ca(OH)2 + heat | Exothermic |
| Redox | ||||
उत्तर
| Reactants | Products | Types of chemical reaction | ||
| 1. | MgH2 | → | Mg + H2 | Oxidation |
| 2. | 2H2S + SO2 | → | 3S + 2H2O | Redox |
| 3. | CaO + H2O | → | Ca(OH)2 + heat | Exothermic |
APPEARS IN
संबंधित प्रश्न
What is wrong with the following chemical equation?
Mg + O → MgO
Correct and balance it.
Complete and balance the following equation:
CA (OH)2 + .............  CaCO3 + H2O
CaCO3 + H2O
Balance the given equation:
Mg(OH)2 + HCI MgCI + H2O
Balance the given equation:
Fe + O2  Fe2O3
Fe2O3
Balance the given equation:
NH3 + CuO  Cu +N2 +H2O
Cu +N2 +H2O
Balance the gievn equation:
NaOH + H2SO4  Na2SO4 + H2O
Na2SO4 + H2O
Fill in the following blank with suitable word:
Chemical equations are balanced to satisfy the law of ............
Convey the following information in the form of a balanced chemical equation:
"An aqueous solution of ferrous sulphate reacts with an aqueous solution of sodium hydroxide to form a precipitate of ferrous hydroxide and sodium sulphate remains in solution."
Give one example of an exothermic reaction.
Is Respiration an endothermic reaction or an exothermic reaction?
When the solution of substance X is added to a solution of potassium iodide, then a yellow solid separates out from the solution.
(a) What do you think substance X is likely to be?
(b) Name the substance which the yellow solid consists of.
(c) Which characteristic of chemical reaction is illustrated by this example?
(d) Write a balanced chemical equation for the reaction which takes place. Mention the physical states of all the reactant and products involved in the chemical equation.
Zinc oxide reacts with carbon, on heating, to form zinc metal and carbon monoxide. Write a balanced chemical equation for this reaction. Name (i) oxidising agent, (ii) reducing agent, in this reaction.
Write balanced chemical equation with state symbols for the following reaction:
Barium chloride solution reacts with sodium sulphate solution to give insoluble barium sulphate and a solution of sodium chloride.
Write word equation for the following skeletal equation:
\[\ce{KClO3 -> KCl + O2}\]
Write your observation for the following chemical reaction and name the product formed :
When dilute acetic acid is poured on baking soda.
Write symbolic representation for the following word equation and balance them :
Calcium oxide + Water → Calcium hydroxide
Complete the following equation:
CH4 + O2 —>
What is a chemical equation? Why it is necessary to balance it?
Write the balanced chemical equation of the following reaction. iron + sulphuric acid → ferrous sulphate + hydrogen.
Write the balanced chemical equations of the following reactions.
chlorine + sulphur dioxide + water → sulphuric acid + hydrogen chloride
Write the balanced chemical equation of the following reaction.
aluminium carbide + water → aluminium hydroxide + methane
Write the balanced chemical equation of the following reaction.
Potassium permanganate + hydrochloric acid → potassium chloride + manganese chloride + chlorine + water
Match column A with column B.
| Column A | Column B |
| (a) Blue salt changes to white and then black | (i) Ammonium dichromate |
| (b) Orange coloured compound changes to green. | (ii) Iodine |
| (c) Red compound changes to brown and then yellow. | (iii) Zinc Nitrate |
| (d) White to yellow when hot and white when cold. | (iv) Copper sulphate |
| (e) Violet solid changes to violet vapours. | (v) Red Lead |
Write word equation for the following molecular equation:
Na2SO3 + 2HCl [dil.] → 2NaCl + H2O + SO2 [g]
Write word equation for the following molecular equation:
\[\ce{N2 + 3H2 ⇌[Fe - 450°C][200 atoms] 2NH3 + \triangle}\]
Word equation:
State why [+ Δ] indication is seen after NH3.
State what 200 atmospheres – indicates.
State the function of Fe in the above reaction.
Write word equation for the following molecular equation:
\[\ce{CuSO4 + 2NaOH -> Na2SO4 + Cu(OH)2↓}\]
Word equation:
State the colour of the products.
Give one example in the case where supplying energy [given below] is necessary for a chemical reaction.
Catalyst
Name the following:
The gas evolved when a dilute acid is added to chalk [limestone].
Write word equation for the following chemical reaction given below. Also state the observation seen in the case.
\[\ce{ZnCo3->[\triangle] ZnO + CO2}\]
Balance the following simple equation:
Al + H2SO4 → Al2(SO4)3 + H2
Write a balanced equation for the following word equation:
Calcium hydroxide + Ammonium chloride → Calcium chloride + Water + Ammonia
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
NaOH + CO2 → Na2CO3 + H2O
Write scientific reason.
Adding zinc particles to a solution of copper sulphate makes the blue solution colorless.
Three beakers labelled as A, B and C each containing 25 mL of water were taken. A small amount of NaOH, anhydrous CuSO4 and NaCl were added to the beakers A, B and C respectively. It was observed that there was an increase in the temperature of the solutions contained in beakers A and B, whereas in case of beaker C, the temperature of the solution falls. Which one of the following statement(s) is(are) correct?
- In beakers A and B, exothermic process has occurred.
- In beakers A and B, endothermic process has occurred.
- In beaker C exothermic process has occurred.
- In beaker C endothermic process has occurred.
What is a product in a chemical equation?
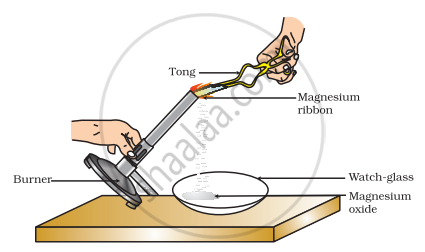
Which of the following is the correct observation of the reaction shown in the above set up?
When aqueous solutions of potassium iodide and lead nitrate are mixed an insoluble substance separates out. The chemical equation for the reaction involved is:
A student took a small amount of copper oxide in a conical flask and added dilute hydrochloric acid to it with constant stirring. He observed a change in colour of the solution.
- Write the name of the compound formed and its colour.
- Write a balanced chemical equation for the reaction involved.
Dil. HCl is added to Zn granules. How will you prove that chemical change has taken place here? Support your response with two arguments.
