Advertisements
Advertisements
प्रश्न
State the main precautions to be taken in finding the latent heat of steam.
उत्तर
The main precautions are:
(i) Every possible care should be taken to see that steam is perfectly dry before passing into water.
(ii) The calorimeter should be properly insulated by keeping it within a wooden box provided with a sliding lid with two holes and lined inside with a layer of cotton wool, cotton pad, etc.
(iii) Steam should no longer be passed when temperature of water in the calorimeter rises approximately by 20°C.
(iv) While passing steam, care must be taken that condensed steam does not escape out.
APPEARS IN
संबंधित प्रश्न
State any two measures to minimize the impact of global warming.
What is the energy absorbed during the phase change called?
Explain the following:
The heat supplied to a substance during it change of state, does not cause any rise in its temperature.
The specific latent heat of fusion of water is ______.
A refrigerator converts 100g of water at 20℃ to ice at – 10℃ in 73.5 min. Calculate the average rate of heat extraction in watt. The specific heat capacity of water is 4.2 J kg-1 K-1, specific latent heat of ice is 336 J g-1 and the specific heat capacity of ice is 2.1 J kg-1 K-1.
Liquid ammonia is used in ice factory for making ice from water. If water at 20°C is to be converted into 2 kg ice at 0°C, how many grams of ammonia are to be evaporated? (Given: The latent heat of vaporization of ammonia = 341 cal/g)
Explain the following temperature vs time graph.
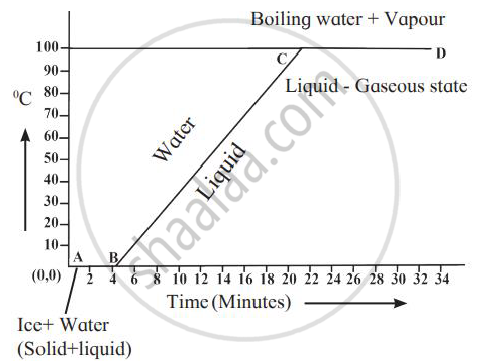
Explain the meaning of the term latent heat. State its S. I. unit.
The specific latent heat of vaporisation of steam is 2260 J/g. Comment on this.
Explain, why no tracks are left on the ice during ice skating?
Why does evaporation causes cooling and why is water used in hot water bottles?
What do you understand by the ‘latent heat of vaporization’ of a substance?
Explain the meaning of greenhouse effect.
1 kg of water is contained in a 1.25 kW kettle. Assuming specific heat capacity of water = 4.2 J/g °C and specific latent heat of vaporization = 2260 J/g, calculate:
(i) the time taken for the temperature of water to rise from 25°C to its boiling point,
(ii) the mass of water which evaporates per minute from the boiling water.
Specific latent heat of vaporisation : J/kg : : specific heat : _______
The latent heat of vaporisation is a term referred for the conversion of gas into liquid.
Write scientific reason.
Use a pressure cooker to cook food in cold air.
Specific latent heat L = ______.
The diagram below shows a cooling curve for a substance:
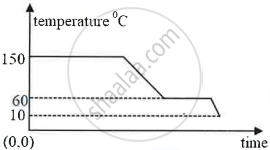
- State the temperatures at which the substance condenses.
- The temperature range in which the substance is in liquid state.
- Why do we prefer ice to ice-cold water for cooling a drink?
