Advertisements
Advertisements
प्रश्न
State the term.
The number of atoms present in a molecule of an element _________
उत्तर
The number of atoms present in a molecule of an element atomicity.
APPEARS IN
संबंधित प्रश्न
Write down the name of a compound represented by the following formula:
K2SO4
Give the name of the element present in the following compound:
Quick lime
Give the name of the element present in the following compound:
Potassium sulphate
Calculate the number of molecules of sulphur (S8) present in 16 g of solid sulphur.
Define: Atomic weight :
Write the formulae of the following compounds. Also name the elements present in them.
- Water
- Ammonia
- Methane
- Sulphur dioxide
- Ethanol
In what form do noble gases occur in nature?
What do the following denote ?
- N
- 2N
- N2
- 2N2
What is the difference between the molecule of an element and the molecule of a compound ? Give one
example of each.
Work out the formula for magnesium hydrogencarbonate.
The cation of an element has :
Molecular compounds are usually formed by the combination between :
Calculate the mass of 3.011 × 1024 atoms of carbon.
Correct the following statement:
A molecule of sulphur is monoatomic.
Why are the symbols and formulae of substances important?
Explain the term – molecules. Give three examples of atoms of the same element forming a molecule. State the atomicity of the same.
Give a reason why –
Helium is considered monoatomic, but hydrogen a diatomic molecule.
Give a reason why –
Ozone is considered triatomic, but phosphorus a tetratomic molecule.
State the atomicity of the following:
Chlorine
State the atomicity of the following:
Argon
Classify the following molecules as the molecules of element or compound
| 1. |  |
|
| 2. |  |
|
| 3. | 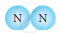 |
|
| 4. | 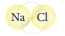 |
Two elements sometimes can form more than one compound.
Give any two examples for heterodiatomic molecules.
Write any two classifications of a molecule.
