Advertisements
Advertisements
प्रश्न
State which of the two a solution of HCl in water or in toluene is an electrolyte. Explain.
उत्तर
When hydrogen chloride gas is dissolved in water, hydrochloric acid is formed. The covalent compound ionises in water because of its polar nature and it can conduct electricity.
\[\ce{HCl + H2O -> H3O+ + Cl-}\]
Hydrogen chloride gas is soluble in toluene, but there is an absence of H3O+ in toluene, so it does not ionise the gas; thus, it cannot conduct electricity.
APPEARS IN
संबंधित प्रश्न
Write an equation for the reactions of hydrochloric acid on manganese (IV) oxide.
Study the flow chart and give balanced equations with conditions for the conversions A, B, C, D and E.
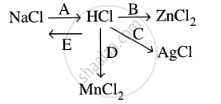
Convert Hydrochloric acid to nascent chlorine.
State which of the two - a solution of HCl in water or in toluene is an electrolyte. Explain.
Convert Hydrochloric acid to nascent chlorine.
State which of the two - a solution of HCl in water or in toluene is an electrolyte. Explain.
State which of the two - a solution of HCl in water or in toluene is an electrolyte. Explain.
Assertion (A): Dry hydrogen chloride gas is collected by the upward displacement of air.
Reason (R): Hydrogen chloride gas is lighter than air.
______ does not form an acid salt.
State which of the two - a solution of \[\ce{HCl}\] in water or in toluene is an electrolyte. Explain.
