Advertisements
Advertisements
प्रश्न
The S.I. unit of specific latent heat is ______.
पर्याय
cal g-1
cal g-1 K-1
J kg-1
J kg -1 K-1
उत्तर
The S.I. unit of specific latent heat is J kg-1.
APPEARS IN
संबंधित प्रश्न
State any two measures to minimize the impact of global warming.
What is the Greenhouse effect?
What do you understand by the term latent heat?
Write the approximate value of specific latent heat of ice.
Which has more heat: 1 g ice at 0℃ or 1g water 0℃? Give reason.
Explain the following:
The heat supplied to a substance during it change of state, does not cause any rise in its temperature.
Answer the following:
Explain the role of latent heat in the change of state of a substance.
Explain the following temperature vs time graph.
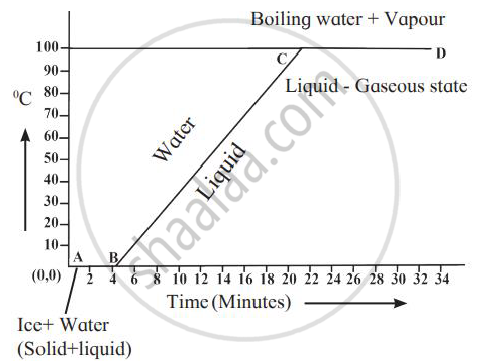
What happens to the heat supplied to a substance when the heat supplied causes no change in the temperature of the substance?
Explain, why no tracks are left on the ice during ice skating?
1 kg of water is contained in a 1.25 kW kettle. Assuming specific heat capacity of water = 4.2 J/g °C and specific latent heat of vaporization = 2260 J/g, calculate:
(i) the time taken for the temperature of water to rise from 25°C to its boiling point,
(ii) the mass of water which evaporates per minute from the boiling water.
When ice is converted into water : constant temperature : : before the water evaporates : _______
Specific latent heat of vaporisation : J/kg : : specific heat : _______
Match the columns.
| Column A | Column B |
| 1) Specific latent heat of fusion | a) Air saturated with vapour |
| 2) Specific latent heat of vaporisation | b) Solid converts into liquid |
| 3) Dew point temperature | c) liquid converts into gas |
Write scientific reason.
Even if boiling water is constantly heated, its temperature does not rise.
20 g of ice at 0°C absorbs 10,920 J of heat energy to melt and change to water at 50°C. Calculate the specific latent heat of fusion of ice. Specific heat capacity of water is 4200 J kg-1 K-1.
