Advertisements
Advertisements
प्रश्न
The trend of which property is represented by the following graph?
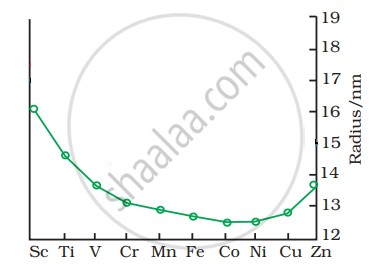
पर्याय
ionization enthalpy
atomic radii
enthalpy of atomization
melting point
उत्तर
Atomic radii
APPEARS IN
संबंधित प्रश्न
Explain why is Fe3+ more stable than Fe2+?
Explain briefly how +2 state becomes more and more stable in the first half of the first row transition elements with increasing atomic number?
Which of the d-block elements may not be regarded as the transition elements?
Give reasons: Sc3+ is colourless in aqueous solution whereas Ti3+ is coloured.
Transition metals with lowest melting point is:
Read the passage given below and answer the following question:
The transition metals when exposed to oxygen at low and intermediate temperatures form thin, protective oxide films of up to some thousands of Angstroms in thickness. Transition metal oxides lie between the extremes of ionic and covalent binary compounds formed by elements from the left or right side of the periodic table. They range from metallic to semiconducting and deviate by both large and small degrees from stoichiometry. Since electron bonding levels are involved, the cations exist in various valence states and hence give rise to a large number of oxides. The crystal structures are often classified by considering a cubic or hexagonal close-packed lattice of one set of ions with the other set of ions filling the octahedral or tetrahedral interstices. The actual oxide structures, however, generally show departures from such regular arrays due in part to distortions caused by packing of ions of different size and to ligand field effects. These distortions depend not only on the number of d-electrons but also on the valence and the position of the transition metal in a period or group.
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
Assertion: Crystal structure of oxides of transition metals often show defects.
Reason: Ligand field effect cause distortions in crystal structures.
Interstitial compounds are formed when small atoms are trapped inside the crystal lattice of metals. Which of the following is not the characteristic property of interstitial compounds?
On strong heating AgNO3, the gases evolved are:-
Which of the following ions acts as a typical transition metal ion?
Which property of transition metals enables them to behave as catalysts?
