Advertisements
Advertisements
प्रश्न
The usefulness of a fertilizer depends upon percentage of nitrogen present in it. Find which of the following is a better fertilizer:
(a) Ammonium nitrate [NH4NO3]
(b) Ammonium phosphate [(NH4)3PO4 (N=14,H=1,O=16,P=31)
उत्तर
(a) Percentage of nitrogen in Ammonium nitrate [NH4NO3]:
(N)2 + (H)4 + (O)3
14 x 2 + 1 x 4 + 3 x 16 = 80.
So, the molecular mass of NH4NO3 = 80.
80 by weight of NH4NO3 contain 28 parts by weight of nitrogen .
100 parts will contain = 28 x 100 /80 = 35‰
So, the percentage composition of nitrogen in Ammonium nitrate is 3535‰
(b) Percentage of nitrogen in Ammonium phosphate [(NH4)3PO4]:
(N)3 + (H)12 +P + (O)4
14 x 3 + 1 x 12+ 31 + 16 x 4 = 149 .
So, the molecular mass of NH4NO3 = 149 .
149 by weight of (NH4)3PO4.contain 42 parts by weight of nitrogen .
100 parts will contain = 42 x 100 /149 = 28.18‰
So, the percentage of nitrogen in ammonium phosphate is 28.18‰
Since the percentage of nitrogen is more in Ammonium nitrate so it is a better fertilizer.
APPEARS IN
संबंधित प्रश्न
Give balanced chemical equations for the following conversions A, B, and C:
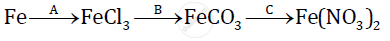
Concentrated nitric acid oxidizes phosphorous to phosphoric acid according to the following equation :
P + 5HNO3 → H3PO4 + H2O + 5NO2
What mass of nitric acid will be consumed at the same time?
Mention the term defined by the following sentence:
The mass of a given volume of gas compared to the mass of an equal volume of hydrogen.
What is the vapour density of ethylene? (Avogadro's number = 6 x 1023; Atomic weight of C = 12, H = 1; Molar volume = 22.4 litres at STP)
A compound 'X' consists of 4.8% of C and 95.2% of Br by mass.
Name the type of chemical reaction by which X can be prepared from ethane.
Give one word or phrase for the following:
Formation of ions from molecules.
Give two tests of the following:
Oxygen
Calculate the relative molecular mass of:
Potassium chlorate
Calculate the number of atoms of each kind in 5.3 grams of sodium carbonate.
67.2 litres of hydrogen combines with 44.8 litres of nitrogen to form ammonia under specific conditions as:
\[\ce{N2_{(g)} + 3H2_{(g)} -> 2NH3_{(g)}}\]
Calculate the volume of ammonia produced. What is the other substance, if any, that remains in the resultant mixture?
