Advertisements
Advertisements
प्रश्न
What are hydrate isomers? Explain with an example.
उत्तर
The exchange of free solvent molecules such as water, ammonia, alcohol, etc., in the crystal lattice with a ligand in the coordination entity, will give different isomers. These types of isomers are called solvate isomers. If the solvent molecule is water, then these isomers are called hydrate isomers.
For example, the complex with the chemical formula \[\ce{CrCl3.+6H2O}\] has three hydrate isomers as shown below.
| \[\ce{[Cr(H2O)6]Cl3}\] | a violet colour compound and gives three chloride ions in solution |
| \[\ce{[Cr(H2O)5Cl]Cl2.H2O}\] | a pale green colour compound and gives two chloride ions in solution |
| \[\ce{[Cr(H2O)4Cl2]Cl.2H2O}\] | dark green colour compound and gives one chloride ion in solution |
APPEARS IN
संबंधित प्रश्न
Choose the most correct option.
Which of the following complexes exist as cis and trans isomers?
1. [Cr(NH3)2Cl4]-
2. [Co(NH3)5Br]2⊕
3. [PtCl2Br2]2- (square planar)
4. [FeCl2(NCS)2]2- (tetrahedral)
The relationship between compound (i) and (ii) is
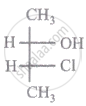 |
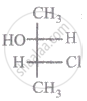 |
| (i) | (ii) |
Complex [COCl2(en)2]+ can
Which of the following compound show optical isomerism?
Which of the following has an optical isomer?
\[\ce{CH3CH2COO- Na+ ->[NaOH, + ?][Heat] CH3CH3 + Na2CO3}\]
Consider the above reaction and identify the missing reagent/chemical.
Write the name of isomerism in the following complexes:
[Cu(NH3)4] [PtCl4] and [Pt(NH3)4] [ CuCl4]
Explain the ionisation isomers.
Name the type of isomerism exhibited by the following pair of compound:
\[\ce{[Cr(H2O)5Cl]Cl2H2O and [Cr(H2O)4Cl2]Cl {.} 2H2O}\]
Which one of the following complex ions has geometrical isomers?
