Advertisements
Advertisements
प्रश्न
What are the limitations of Rutherford’s model of the atom?
उत्तर
(i) An electron orbiting around the nucleus is accelerated towards it. An accelerating charged particle must produce radiation and lose energy. Thus, electrons in an atom must constantly emit radiation and lose energy. Because of this energy loss, the electron will slow down and cannot withstand the nucleus' attraction. As a result, the electron should spiral and eventually fall into the nucleus (see image).
If this occurs, the atom should collapse in approximately 10-8 seconds. However, this does not occur; atoms are stable. This suggests that something is amiss with Rutherford's nuclear model of atoms.
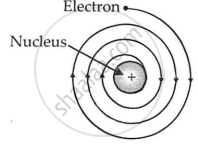
(ii) Rutherford's atomic model says nothing about how electrons are arranged in an atom.
APPEARS IN
संबंधित प्रश्न
Rutherford’s alpha-particle scattering experiment was responsible for the discovery of ______.
Name the particles used by Rutherford in his experiment on the discovery of nucleus. Also state the charge on these particles.
Rutherford's alpha particle scattering experiment led to the discovery of :
What conclusions were made from the observations of Gold foil experiment?
Which of the following statements about Rutherford’s model of atom are correct?
(i) considered the nucleus as positively charged
(ii) established that the α–particles are four times as heavy as a hydrogen atom
(iii) can be compared to solar system
(iv) was in agreement with Thomson’s model
Why did Rutherford select a gold foil in his α–ray scattering experiment?
Which of the following conclusions could not be derived from Rutherford’s α -particle scattering experiment?
To study the internal structure of the atom, Rutherford used ______.
In Geiger - Marsden scattering experiment, the trajectory traced by an α-particle depends on ______.
The Rutherford α-particle experiment shows that most of the α-particles pass through almost unscattered. While some are scattered through large angles. What information does it give about the structure of the atom?
