Advertisements
Advertisements
प्रश्न
What is the difference between the chemical composition of soaps and detergents? State in brief the action of soaps in removing an oily spot from a shirt. Why are sops not considered suitable for washing where water is hard?
उत्तर
Soaps are potassium or sodium salts of long chain carboxylic acid. On the other hand, detergents are ammonium or sulphonate salts of long chain carboxylic acid.
Action of soap in removing an oily spot from a shirt
The dirt present on clothes is organic in nature and insoluble in water. Therefore, it cannot be removed by only washing with water. When soap is dissolved in water, its hydrophobic ends attach themselves to the dirt and remove it from the cloth. Then, the molecules of soap arrange themselves in micelle formation and trap the dirt at the centre of the cluster. These micelles remain suspended in the water. Hence, the dust particles are easily rinsed away by water.
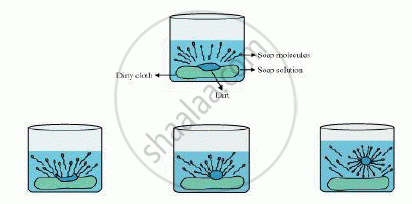
Soap does not work properly when the water is hard.
A soap is a sodium or potassium salt of long chain fatty acids. Hard water contains salts of calcium and magnesium. When soap is added to hard water, calcium and magnesium ions present in water displace sodium or potassium ions from the soap molecules forming an insoluble substance called scum. A lot of soap is wasted in the process.
संबंधित प्रश्न
A student takes four test tubes marked P, Q, R and S of 25 mL capacity and fills 10 mL of distilled water in each. He dissolves one spoon full of four different salts in each as − KCl in P, NaCl in Q, CaCl2 in R and MgCl2 in S. He then adds about 2 mL of a sample of soap solution to each of the above test tubes. On shaking the contents of each of the test tubes, he is likely to observe a good amount of lather (foam) in the test tubes marked
(a) P and Q
(b) R and S
(c) P, Q and R
(d) P, Q and S
What change will you observe if you test soap with litmus paper (red and blue)?
A student requires hard water for an experiment in his laboratory which is not available in the neighbouring area. In the laboratory there are some salts, which when dissolved in distilled water can convert it into hard water. Select from the following groups of salts, a group, each salt of which when dissolved in distilled water will make it hard.
(A) Sodium chloride, Potassium chloride
(B) Sodium sulphate, Potassium sulphate
(C) Sodium sulphate, Calcium sulphate
(D) Calcium sulphate, Calcium chloride
While studying the saponification reaction, what do you observe when you mix an equal amount of colourless vegetable oil and 20% aqueous solution of NaOH in a beaker?
(A) The colour of the mixture has become dark brown
(B) A brisk effervescence is taking place in the beaker
(C) The outer surface of the beaker has become hot
(D) The outer surface of the beaker has become cold
What is saponification? Write the chemical equation of the reaction involved in this process. Name all the substances which take part in this process and also those which are formed.
Why are soaps not suitable for washing clothes when the water is hard?
Explain the action of soap in removing an oily spot from a piece of cloth.
What is meant by 'surface activity'?
What are the differences between Soap and synthetic detergent?
The soap molecule has a
