Advertisements
Advertisements
प्रश्न
What is the difference between the chemical composition of soaps and detergents? State in brief the action of soaps in removing an oily spot from a shirt. Why are sops not considered suitable for washing where water is hard?
उत्तर
Soaps are potassium or sodium salts of long chain carboxylic acid. On the other hand, detergents are ammonium or sulphonate salts of long chain carboxylic acid.
Action of soap in removing an oily spot from a shirt
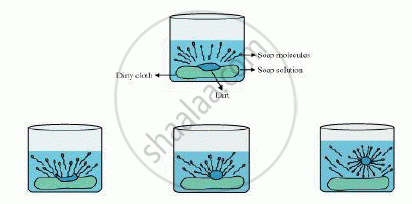
The dirt present on clothes is organic in nature and insoluble in water. Therefore, it cannot be removed by only washing with water. When soap is dissolved in water, its hydrophobic ends attach themselves to the dirt and remove it from the cloth. Then, the molecules of soap arrange themselves in micelle formation and trap the dirt at the centre of the cluster. These micelles remain suspended in the water. Hence, the dust particles are easily rinsed away by water.
Soap does not work properly when the water is hard.
A soap is a sodium or potassium salt of long chain fatty acids. Hard water contains salts of calcium and magnesium. When soap is added to hard water, calcium and magnesium ions present in water displace sodium or potassium ions from the soap molecules forming an insoluble substance called scum. A lot of soap is wasted in the process.
संबंधित प्रश्न
Describe in brief the cleansing action of soap.
Fill in the following blank with suitable word:
The sodium salt of a long chain fatty acid is called .............
What is a soap? Name one soap.
Why are soaps not suitable for washing clothes when the water is hard?
Differentiate between:
Detergents and Soaps.
Soap is a salt of ______ and sodium hydroxide.
How will you check with the help of soap powder whether water is hard?
What is meant by 'surface activity'?
What are the differences between Bath soap and soap for washing clothes?
The saponification of a fat or oil is done using _____ solution for hot process.
