Advertisements
Advertisements
प्रश्न
What energy change would you Expect to take place in the molecules of a substance when it undergoes:
(i) a change in its temperature?
(ii) a change in itsstate without any change in its temperature?
उत्तर
(i) Inter molecular space changes.
(ii) Intermolecular space increases.
APPEARS IN
संबंधित प्रश्न
What is the principle of Calorimetry?
Define calorimetry ?
200 g of hot water at 80°C is added to 400 g of cold water at 10°C. Neglecting the heat taken by the container, calculate the final temperature of the mixture of water. Specific heat capacity of water = 4200 J kg-1K-1.
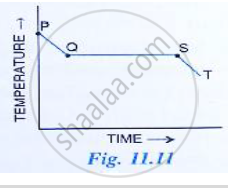
Fig 11. 11 shows the variation in temperature with time when some wax cools from the liquid phase to the solid phase.
(i) In which part of the curve, the wax is in liquid phase?
(ii) What does the part QS of the curve represent?
(iii) In which part of the curve, the wax will be the in the liquid as well as solid phase?
(iv) In which part of the curve, the wax is in solid phase?
The specific heat capacity of a substance A is 3,800 Jkg `"^(–1)K^(–1)` and that of a substance B is 400 `"^(–1)K^(–1)`. Which of the two substances is a good conductor of heat ? Give a reason for your answer.
A piece of copper of mass 1 kg is dropped into 2 kg of water at 15°C. If the final temperature of the mixture is 40°C, calculate the intial temperature of copper.
Fill in the following blank using suitable word:
The normal temperature of a human body is .............
Ice is more effective in cooling than the ice-water. Explain.
A Bunsen burner raises the temperature of 500g of water from 10°C to 100°C in 5 minutes. What heat is supplied per second?
