Advertisements
Advertisements
प्रश्न
What happens when ethanoic acid reacts with sodium hydroxide? Write equation of the reaction involved.
Write chemical equation for the reaction of ethanoic acid with sodium hydroxide.
उत्तर
The reaction between ethanoic acid \[\ce{(CH3COOH)}\] and sodium hydroxide \[\ce{(NaOH)}\] is a neutralisation reaction:
\[\ce{CH3COOH + NaOH → CH3COONa + H2O}\]
In this equation, ethanoic acid reacts with sodium hydroxide to produce sodium acetate \[\ce{(CH3COONa)}\] and water \[\ce{(H2O)}\]. This is a typical acid-base neutralization process, where an acid combines with a base to form salt and water.
संबंधित प्रश्न
Consider the following comments about saponification reactions:
I. Heat is evolved in these reactions.
II. For quick precipitation of soap, sodium chloride is added to the reaction mixtures.
III. Saponification reactions are a special kind of neutralisation reactions.
IV. Soaps are basic salts of long-chain fatty acids.
The correct comments are
(a) I, II and III
(b) II, III and IV
(c) I, II and IV
(d) Only I and IV
When ethanoic acid reacts with sodium hydrogen carbonate, then a salt X is formed and a gas Y is evolved. Name the salt X and gas. Y Describe an activity with the help of a labelled diagram of the apparatus used to prove that the evolved gas is the one which you have named. Also write the chemical equation of the reaction involved.
A student adds 4 mL of acetic acid to a test tube containing 4 mL of distilled water. He then shakes the test tube and leaves it to settle. After about 10 minutes he observes:
(A) a layer of water over the layer of acetic acid
(B) a layer of acetic acid over the layer of water
(C) a precipitate settling at the bottom of the test tube
(D) a clear colourless solution
Explain the following term with example.
Reduction
Write a balanced equation for the following:
Write the equation for the preparation of ethylene from ethyl alcohol.
Write a balanced chemical equation for the following:
Monochloroethane is hydrolysed with aqueous KOH.
Convert ethane to acetic acid.
Explain the following reaction with an example.
Saponification
Observe the diagram given below and answer the questions:
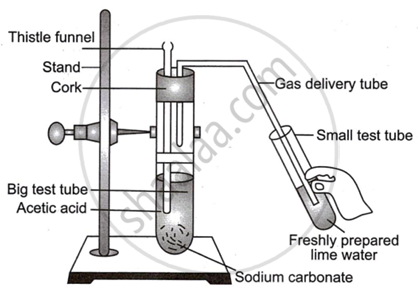
- Name the reactants in this reaction.
- Which gas comes out as effervescence in the bigger test tube?
- What is the colour change in the lime water?
- In the above experiment instead of sodium carbonate which chemical can be used to get same products?
- Write the use of acetic acid.
