Advertisements
Advertisements
प्रश्न
Explain the following term with example.
Reduction
उत्तर
A chemical reaction in which a substance gains hydrogen or loses oxygen is called a reduction reaction.
E.g. In the following reaction, ethylene (ethene) is reduced to ethane.
\[\ce{\underset{\text{Ethylene}}{H2C = CH2} ->[H2][Pt/Ni] \underset{\text{Ethane}}{CH3CH3}}\]
संबंधित प्रश्न
The molecular formula of acetic acid is _________ .
(a) CH3COOH
(b) CH3 – CH3
(c) C6H6
(d) C2H4
When ethanol reacts with ethanoic acid in the presence of conc. H2SO4, a substance with fruity smell is produced. Answer the following:-
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of conc. H2SO4 in the reaction.
List two uses of esters.
Complete the following chemical equations : C2H5OH + Na →
Give balanced chemical equations for Sodium ethanoate to methane.
Write the formulae of ethanoic acid.
Fill in the following blank with suitable word:
The next higher homologue of ethanol is ...............
If you take a pinch of sodium hydrogen carbonate powder in a test-tube and add drop-by-drop acetic acid to it, what would you observe immediately? List any two main observations.
Which one of the following are the correct observations about acetic acid?
(A) It turns blue litmus red and smells like vinegar
(B) It turns blue litmus red and smells like burning sulphur
(C) It turns res litmus blue and smells like vinegar
(D) It turns red litmus blue and has a fruity smell
Explain the following term with an example.
Oxidant
State how the following conversions can be carried out:
Ethene to Ethyl alcohol
Distinguish between esterification and saponification reactions with the help of chemical equations for each.
With a labelled diagram describe in brief an activity to show the formation of ester.
Write the molecular formula of the given compound.
Sodium ethanoate
Explain the following reaction with an example.
Saponification
What are catalysts?
The reaction between acetic acid and sodium carbonate is shown in the following figure.
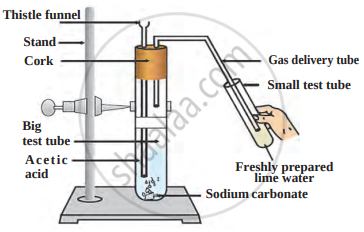
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
A spatula full of sodium carbonate is taken in a test tube and 2 mL of dilute ethanoic acid is added to it.
Write a chemical equation for the reaction.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
