Advertisements
Advertisements
प्रश्न
What is ferromagnetism?
Explain the following with suitable examples:
Ferromagnetism
उत्तर १
The substances that can be permanently magnetised even in the absence of a magnetic field are called ferromagnetic substances, and the mechanism is called ferromagnetism.
उत्तर २
The substances that are strongly attracted by a magnetic field are called ferromagnetic substances. Ferromagnetic substances can be permanently magnetised even in the absence of a magnetic field. Some examples of ferromagnetic substances are iron, cobalt, nickel, gadolinium, and CrO2.
In solid state, the metal ions of ferromagnetic substances are grouped together into small regions called domains, and each domain acts as a tiny magnet. In an unmagnetized piece of a ferromagnetic substance, the domains are randomly oriented, and so their magnetic moments get cancelled. However, when the substance is placed in a magnetic field, all the domains get oriented in the direction of the magnetic field. As a result, a strong magnetic effect is produced. This ordering of domains persists even after the removal of the magnetic field. Thus the ferromagnetic substance becomes a permanent magnet.

Schematic alignment of magnetic moments in ferromagnetic substances
APPEARS IN
संबंधित प्रश्न
Iron (z=26) is highly ferromagnetic. Explain.
What type of magnetism is shown in the following alignment of magnetic moments?

What type of magnetism is shown by a substance if magnetic moments of domains are arranged in same direction?
What type of substances would make better permanent magnets, ferromagnetic or ferrimagnetic. Justify your answer.
Analysis shows that nickel oxide has the formula Ni0.98O1.00. What fractions of nickel exist as Ni2+ and Ni3+ ions?
Explain the following with suitable examples: Paramagnetism
Explain the following with suitable examples: Ferrimagnetism
Give reasons:Ferrimagnetic substances show better magnetism than antiferromagnetic substances.
The complexion [Ni(CN)4]2- is:
Explain why:
(i) Transition elements form coloured compounds.
(ii) Interhalogen compounds are more reactive than their constituent elements.
(iii) Cu+ is diamagnetic but Cu2+ is paramagnetic. (Z = 29)
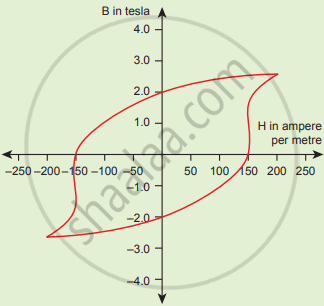
The BH curve for a ferromagnetic material is shown in the figure. The material is placed inside a long solenoid which contains 1000 turns/cm. The current that should be passed in the solenonid to demagnetize the ferromagnet completely is
What is magnetic susceptibility?
Compare dia, para and ferromagnetism.
Give the properties of dia/para/ferromagnetic materials.
Example of ferromagnetic substance is ____________.
Substances which are weakly repelled in magnetic field are known as ____________.
All those atoms or molecules which have an odd number of electrons are
When heated to high temperature, ferromagnetic substance changes to ____________.
Which type of substances would make better permanent magnets?
Which of the following arrangements shows schematic alignment of magnetic moments of antiferromagnetic substances?
A ferromagnetic substance becomes a permanent magnet when it is placed in a magnetic field becuase ______.
The value of magnetic moment is zero in the case of antiferromagnetic substances because the domains:
(i) get oriented in the direction of the applied magnetic field.
(ii) get oriented opposite to the direction of the applied magnetic field.
(iii) are oppositely oriented with respect to each other without the application of magnetic field.
(iv) cancel out each other’s magnetic moment.
Which of the following statements are correct?
(i) Ferrimagnetic substances lose ferrimagnetism on heating and become paramagnetic.
(ii) Ferrimagnetic substances do not lose ferrimagnetism on heating and remain ferrimagnetic.
(iii) Antiferromagnetic substances have domain structures similar to ferromagnetic substances and their magnetic moments are not cancelled by each other.
(iv) In ferromagnetic substances all the domains get oriented in the direction of magnetic field and remain as such even after removing magnetic field.
Which one of the following would feel attraction when placed in magnetic field: Co2+, Ag+, Ti4+, Zn2+
Which one of the following pairs has only paramagnetic species?
Which of the following is not a ferroelectric compound?
Given below are two statements labelled as Assertion (A) and Reason (R).
Assertion: Magnetic moment values of actinides are lesser than the theoretically predicted values.
Reason: Actinide elements are strongly paramagnetic.
Select the most appropriate answer from the options given below:
Magnetic materials used for making permanent magnets (P) and magnets in a transformer (T) have different properties of the following, which property best matches for the type of magnet required ?
The correct order of bond strength is ______.
Which of the following statements are correct?
- Electric monopoles do not exist whereas magnetic monopoles exist.
- Magnetic field lines due to a solenoid at its ends and outside cannot be completely straight and confined.
- Magnetic field lines are completely confined within a toroid.
- Magnetic field lines inside a bar magnet are not parallel.
- χ = -1 is the condition for a perfect diamagnetic material, where x is its magnetic susceptibility.
The susceptibility of a paramagnetic material is 99. The permeability of the material in Wb/A-m is ______.
[permeability of the free space μ0 = 4π × 10-7 Wb/A - m]
The value of aluminium susceptibility is 2.2 × 10-5. The percentage increase in the magnetic field if space within a current carrying toroid is filled with aluminium is `"x"/10^4`. Then the value of x is ______.
Among the following ions, which one has the highest paramagnetism?
Which of the following is not paramagnetic?
Which one of the following compounds is diamagnetic and colourless?
The metal complex ion that is paramagnetic is ______.
(Atomic number of Fe = 26, Cu = 29, Co = 27 and Ni = 28)
