Advertisements
Advertisements
प्रश्न
What is the hybridisation of each carbon in H2C = C = CH2.
उत्तर
In \[\ce{H2\overset{(1)}{C} = \overset{(2)}{C} = \overset{(3)}{C}H2}\], carbon (1) and (3) are sp2 hybridised and carbon (2) is sp hybridized.
APPEARS IN
संबंधित प्रश्न
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5OH
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5NO2
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
CH3CH = CHCHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
C6H5 – CHO
Draw the resonance structure for the following compound. Show the electron shift using curved-arrow notation.
\[\ce{CH3CH = CH\overset{+}{C}H2}\]
In which of the following representations given below spatial arrangement of group/ atom different from that given in structure ‘A’?
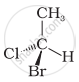 |
| (A) |
| (i) | 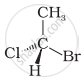 |
| (ii) | 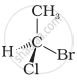 |
| (iii) | 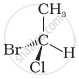 |
| (iv) | 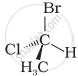 |
Show the polarisation of carbon-magnesium bond in the following structure.
CH3 – CH2 – CH2 – CH2 – Mg – X
Draw the possible resonance structures for \[\ce{CH3 - \overset{\bullet\bullet}{\underset{\bullet\bullet}{O}} - \overset{+}{C}H2}\] and predict which of the structures is more stable. Give reason for your answer.
Resonance structures of propenal are given below. Which of these resonating structures is more stable? Give reason for your answer.
\[\ce{\underset{I}{CH2 = CH - CH = O} <-> \underset{II}{\overset{⊕}{C}H2 - CH = CH - \overset{Θ}{O}}}\]
Which of the two structures (A) and (B) given below is more stabilised by resonance? Explain.
\[\ce{\underset{(A)}{CH3COOH}}\] and \[\ce{\underset{(B)}{CH3CO\overset{Θ}{O}}}\]
