Advertisements
Advertisements
प्रश्न
When 1 mol \[\ce{CrCl3.6H2O}\] is treated with excess of \[\ce{AgNO3}\], 3 mol of \[\ce{AgCl}\] are obtained. The formula of the complex is ______.
पर्याय
\[\ce{[CrCl3 (H2O)3].3H2O}\]
\[\ce{[CrCl2 (H2O)4]Cl.2H2O}\]
\[\ce{CrCl(H2O)5]Cl2.H2O}\]
\[\ce{[Cr(H2O)6]Cl3}\]
उत्तर
When 1 mol \[\ce{CrCl3.6H2O}\] is treated with excess of \[\ce{AgNO3}\], 3 mol of \[\ce{AgCl}\] are obtained. The formula of the complex is \[\ce{[Cr(H2O)6]Cl3}\].
Explanation:
One mol of \[\ce{AgCl}\] is precipitated by one mole of \[\ce{Cl^-}\], therefore three moles of \[\ce{AgCl}\] would get precipitared by three moles of chloride ions, \[\ce{Cl^-}\] and in this case \[\ce{3Cl^-}\] are present in ionization sphere (i.e. out side the coordination sphere) in complex at \[\ce{[Cr(H2O)6]Cl3}\], therefore when 1 mol of it is treated with excess of this complex 3 mols of \[\ce{AgCl}\] is precipitated.
APPEARS IN
संबंधित प्रश्न
Give the oxidation state, d-orbital occupation and coordination number of the central metal ion in the following complex:
cis-[CrCl2(en)2]Cl
Which of the following compounds is used as a semipermeable membrane?
(a) Potassium ferrocyanide
(b) Potassium argentocyanide
(c) Sodium meta aluminate
(d) Copper ferrocyanide
Write applications of co-ordination compounds in medicine and electroplating.
Write structures of compounds A, B and C in of the following reactions
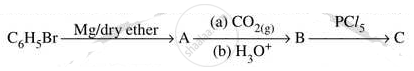
Write structures of compounds A, B and C in of the following reactions
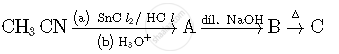
Which of the following is an ionic ligand?
Which one of the following ligands forms a chelate?
The equivalents of ethylene diamine required to replace the neutral ligands from the coordination sphere of the trans-complex of CoCl3.4NH3 is ______. (Round off to the Nearest Integer).
Glycinato ligand is ______.
What is meant by didentate ligand?
