Advertisements
Advertisements
प्रश्न
When metal X is treated with a dilute acid Y, then a gas Z is evolved which burns readily by making a little explosion.
(a) Name any two metals which can behave like metal X.
(b) Name any two acids which can behave like acid Y.
(c) Name the gas Z.
(d) Is the gas Z lighter than or heavier than air?
(e) Is the reaction between metal X and dilute acid Y, exothermic or endothermic?
(f) By taking a specific example of metal X and dilute acid Y, write a balanced chemical equation for the reaction which takes place. Also indicate physical states of all the reactants and products.
उत्तर
(a) Zinc (Zn) and magnesium (Mg) metals can behave like metal X.
(b) Sulphuric acid (H2SO4) and hydrochloric acid (HCl) can behave like acid Y.
(c) Gas Z is hydrogen gas (H2).
(d) Gas Z (i.e., hydrogen) is lighter than air because it is the lightest element in the periodic table.
(e) The reaction between metal X and dilute acid Y is exothermic because it produces huge amount of heat.
(f) If X is zinc and Y is sulphuric acid, then the equation can be written as follows:
Zn (s) + H2SO4 (aq) → ZnSO4 (aq) + H2 (g)
APPEARS IN
संबंधित प्रश्न
Balance the following chemical equation.
\[\ce{NaOH + H2SO4 -> Na2SO4 + H2O}\]
Balance the given equation:
Fe + O2  Fe2O3
Fe2O3
Balance the following chemical equation:
MnO2 + HCI → MnCI2 + CI2 + H2O
Write the balanced equation for the following chemical reaction.
\[\ce{Barium chloride + Aluminium sulphate -> Barium sulphate + Aluminium chloride}\]
Write your observation for the following chemical reaction and name the product formed :
When dilute acetic acid is poured on baking soda.
Write the balanced chemical equation of the following reaction.
ammonia + oxygen → nitric oxide + water
Write word equation for the following chemical reaction given below. Also state the observation seen in the case.
\[\ce{2SO2 + O2 ⇌[V2O5][450°C] 2SO3}\]
Underline the compound in the equation given below, it is incorrectly balanced and write the correct balancing for the same.
CaC2 + N2 → 2CaCN2 + C
Give the characteristic tests for the following gases :
- CO2
- SO2
- O2
- H2
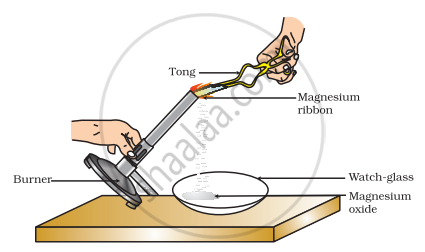
Which of the following is the correct observation of the reaction shown in the above set up?
