Advertisements
Advertisements
प्रश्न
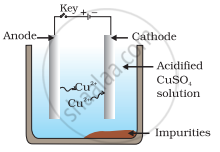
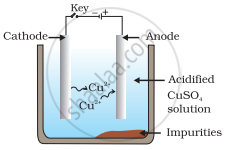
Which one of the following figures correctly describes the process of electrolytic refining?
पर्याय
उत्तर

In the process of electrolytic refining of copper, the impure copper metal is made the anode and connected to positive terminal of the battery. A thin strip of pure copper metal is made the cathode and connected to negative terminal of the battery. A solution of the metal salt (CuSO4) is used as an electrolyte. On passing the current through the electrolyte, the pure metal from the anode dissolves into the electrolyte as Cu2+. An equivalent amount of pure metal from the electrolyte is deposited on the cathode. The soluble impurities go into the solution, whereas, the insoluble impurities settle down at the bottom of the anode and are known as anode mud.
APPEARS IN
संबंधित प्रश्न
What is meant by refining of a metal? Name the most widely used method for the refining of impure metals obtained by various reduction processes. Describe this method with the help of a labelled diagram by taking the example of any metal.
During the refining of an impure metal by electrolysis, the pure metal is a deposited:
(a) at cathode
(b) on the walls of electrolytic tank
(c) at anode
(d) at the bottom of electrolytic tank
Name one metal each occurring as :
A carbonate
Name one metal each occurring as :
An oxide
Ionic compounds are electrically _______.
Electrolysis is used to obtain pure metal from impure metal.
What are the constituents of solder alloy? Which property of solder makes it suitable for welding electrical wires?
Identify the terms for the following:
The electrode where reduction takes place.
Answer the following question with reference to the electrorefining of copper:
What do you observe at the cathode?
Answer the following questions with reference to the electrorefining of copper:
Write the reaction taking place at the cathode.