Advertisements
Advertisements
प्रश्न
Why does iron or zinc not occur free in nature?
उत्तर
Iron and zinc are quite reactive, and hence, they do not occur in the free state. Metal compounds found in nature are their oxides, carbonates and sulphides.
APPEARS IN
संबंधित प्रश्न
Why are alkali metals kept in kerosene oil?
Metal A has an electronic configuration of 2, 8, 1 and metal B has 2, 8, 8, 2 which is a more reactive metal.
Give the effect of heat on their: carbonates
Metal A has an electronic configuration of 2, 8, 1 and metal B has 2, 8, 8, 2 which is a more reactive metal.
Give the effect of heat on their: nitrates
Explain the following terms:
(a) flux
An ore on being heated in air forms sulphurous anhydride. Write the process used for the concentration of this ore.
On which factors does the purification of metals depend?
Which metal is used for:
lithographic plates for printing
Aluminium is extracted from its chief ore, bauxite. The ore is first purified and then the metal is extracted from it by electrolytic reduction.
Write three balanced equations for the purification of bauxite.
Complete the following flow chart and answer the questions below:
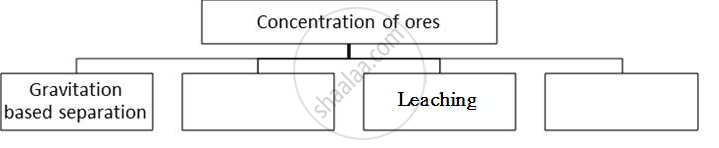
i) In which method pine oil is used?
ii) Explain what method of concentration in brief.
A compound that is added to lower the fusion temperature of the electrolytic bath in the extraction of aluminium.
