Advertisements
Advertisements
प्रश्न
Write down the IUPAC name of the following complex and indicate the oxidation state, electronic configuration and coordination number. Also, give the stereochemistry and magnetic moment of the complex:
Cs[FeCl4]
उत्तर
| IUPAC name of the hybrid compound | Oxidation state of the central metal | Coordination number | Shape | Central metal ion | Electronic configuration | The value of n and the magnetic moment | Stereochemistry |
| Caesium tetrachloridoferrate(III) | +1 + x + 4 × (−1) = 0 ∴ x = +3 |
4 | Tetrahedral | Fe3+ | 3d5, \[\ce{t^3_{2g}e^2_g}\] | n = 5, `sqrt(5(5 + 2))` = 5.92 BM |
Optically inactive |
APPEARS IN
संबंधित प्रश्न
What is the IUPAC name of 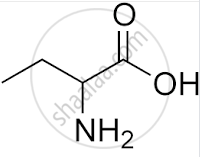
Write the IUPAC name of 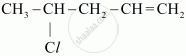
Write the IUPAC names of the following coordination compounds: [Cr(NH3)3Cl3]
When a coordination compound CoCl3.6NH3 is mixed with AgNO3, 3moles of AgCl are precipitated per mole of the compound. Write (ii) IUPAC name of the complex
Write down the IUPAC name of the following complex: [Co(NH3)5 (NO2)](NO3)2
When a co-ordination compound CrCl3.6H2O is mixed with AgNO3, 2 moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex.
(ii) IUPAC name of the complex.
Write the IUPAC name of the complex [Cr(NH3)4 Cl2]Cl.
Specify the oxidation number of the metal in the following coordination entity:
[Co(H2O)(CN)(en)2]2+
Using IUPAC norms write the systematic name of the following:
[Pt(NH3)2Cl(NH2CH3)]Cl
Using IUPAC norms, write the systematic name of the following:
[NiCl4]2−
Using IUPAC norms, write the systematic name of the following:
[Ni(NH3)6]Cl2
Write down the IUPAC name of the following complex and indicate the oxidation state, electronic configuration and coordination number. Also, give the stereochemistry and magnetic moment of the complex:
[Co(NH3)5Cl]Cl2
The oxidation number of cobalt in K[Co(CO)4] is
(i) +1
(ii) +3
(iii) −1
(iv) −3
Identify 'A' and 'B' and rewrite the reactions
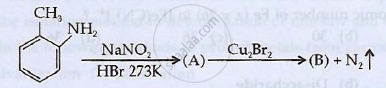
Write the IUPAC name of the following coordination compound:
K3[Fe(CN)6]
Write the IUPAC names of the following coordination compounds:
[CoBr2(en)2]+, (en = ethylenediamine)
Write the IUPAC name of the compound
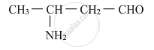
Name the type of isomerism shown by the following compounds:
[Co(Pn)2Cl2]+ and [Co(en)2Cl2]+
Which of the following is paramagnetic?
The formula Co(NH3)5CO3Cl could represent a carbonate or a chloride. Write the structures and names of possible isomers.
