Advertisements
Advertisements
प्रश्न
The oxidation number of cobalt in K[Co(CO)4] is
(i) +1
(ii) +3
(iii) −1
(iv) −3
उत्तर
We know that CO is a neutral ligand and K carries a charge of +1.
Therefore, the complex can be written as K+[Co(CO)4]−. Therefore, the oxidation number of Co in the given complex is −1.
APPEARS IN
संबंधित प्रश्न
Write the structure and IUPAC names of isomeric aldehydes having molecular formula C5H10O.
IUPAC name of K4[Fe(CN)6] is
Write the IUPAC name of the given compound:
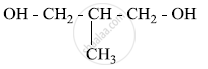
Write the IUPAC name of the following coordination compound:
[Co(NH3)6]Cl3
Write the IUPAC name of the following coordination compound:
K2[PdCl4]
Specify the oxidation number of the metal in the following coordination entity:
[CoBr2(en)2]+
Specify the oxidation number of the metal in the following coordination entity:
[Cr(NH3)3Cl3]
Using IUPAC norms, write the systematic name of the following:
[Mn(H2O)6]2+
Write down the IUPAC name of the following complex and indicate the oxidation state, electronic configuration and coordination number. Also, give the stereochemistry and magnetic moment of the complex:
[CrCl3(py)3]
Write down the IUPAC name of the following complex and indicate the oxidation state, electronic configuration and coordination number. Also, give the stereochemistry and magnetic moment of the complex:
Cs[FeCl4]
Write down the IUPAC name of the following complex and indicate the oxidation state, electronic configuration and coordination number. Also, give the stereochemistry and magnetic moment of the complex:
K4[Mn(CN)6]
Identify 'A' and 'B' and rewrite the reactions

Write the IUPAC names of the following coordination compounds:
[CoBr2(en)2]+, (en = ethylenediamine)
Write the IUPAC name of the compound.
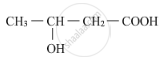
How will you convert the following?
Nitrobenzene into aniline
Write the IUPAC name of the compound
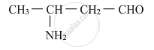
Write the IUPAC name of the K3[Fe(C2O4)3].
Which of the following is paramagnetic?
The formula Co(NH3)5CO3Cl could represent a carbonate or a chloride. Write the structures and names of possible isomers.
Write the formula for the following coordination compound.
Bis (ethane-1,2-diamine) dihydroxidochromium (III) chloride
