Advertisements
Advertisements
प्रश्न
Write preparation of low density polythene.
उत्तर
Preparation of low density polythene:
- LDP is prepared by polymerization of ethylene under high pressure (1000 – 2000 atm) and temperature (350 – 570 K) in presence of traces of O2 or peroxide as initiator.
\[\ce{{n} CH2 = CH2 ->[Traces of O2 or][peroxide at 350–570 K, 1000–2000 atm] LDP}\] - The mechanism of this reaction involves free radical addition and H-atom abstraction. The latter results in branching.
- Polymeric chains are loosely held due to branching and the polymer has low density.
APPEARS IN
संबंधित प्रश्न
Write any ‘two' uses of terylene.
Write the reactions involved in the preparation of PVC
Write the structures of the monomers used for getting the following polymers
Melamine – formaldehyde polymer
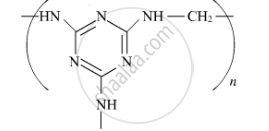
Write the monomers of the following polymer :
Choose the correct option from the given alternatives.
Which of the following is made up of polyamides?
Answer the following in one sentence.
Identify thermoplastic and thermosetting plastic from the following:
- PET
- Urea formaldehyde resin
- Polythene
- Phenol formaldehyde resin
Answer the following.
Write the reaction of the formation of Nylon 6.
Answer the following.
Write the reaction of the formation of Terylene.
Answer the following.
Name the polymer type in which following linkage is present.
\[\begin{array}{cc}\ce{- C - O -}\\||\phantom{.....}\\
\ce{O\phantom{.....}}\end{array}\]
Answer the following.
Match the following pairs:
| Name of polymer | Monomer |
| 1. Teflon | a. CH2 = CH2 |
| 2. PVC | b. CF2 = CF2 |
| 3. Polyester | c. CH2 = CHCl |
| 4. Polythene | d. C6H5OH and HCHO |
| 5. Bakelite | e. Dicarboxylic acid and polyhydoxyglycol |
Draw the structures of polymers formed from the following monomers
\[\ce{n HOOC–R–COOH + n HO–R'–OH}\]
Identify condensation polymers and addition polymers from the following.
\[\begin{array}{cc}\ce{-(CH2 - CH -)_{n}}\\
\phantom{....}|\\\ce{\phantom{.......}C6H5}
\end{array}\]
Attempt the following:
Explain the vulcanisation of rubber. Which vulcanizing agents are used for the following synthetic rubber?
a. Neoprene
b. Buna-N
Attempt the following:
Write preparation, properties and uses of Teflon.
Answer the following.
Write main specialities of Buna-S, Neoprene rubber?
Write the name of the catalyst used for preparation of high density polythene polymer.
Mention two uses of LDP.
Write chemical reaction for preparation of the following.
Buna-S
Write the name of one example of each polymer in which following repeating units.
\[\begin{array}{cc}
\ce{(-CF2-CF2-), -[NH-(CH2)5-CO] -, -(CH2-CH-), (-CH2-CH2-)}\\
\phantom{............................}|\\
\phantom{..............................}\ce{CN}
\end{array}\]
Write chemical reactions for the preparation of high-density polythene.
Write two uses and two properties of polythene.
The following structure represents the polymer:
\[\begin{array}{cc}
\ce{[-C-CH2-NH-C-(-CH2)5 NH -]_{{n}}}\\
\phantom{}||\phantom{.............}||\phantom{................}\\
\phantom{}\ce{O}\phantom{.............}\ce{O}\phantom{................}
\end{array}\]
Identify additional polymers from the following.
I. \[\begin{array}{cc}
\ce{-(CH2 - CH -)_{{n}}}\\
\phantom{....}|\\
\phantom{.......}\ce{C6H5}
\end{array}\]
II. \[\ce{-(CH2 - CH = CH - CH2 -)_{{n}}}\]
III. \[\ce{-(CO(CH2)4 - CONH(CH2)6NH -)_{{n}}}\]
IV.
![]()
Which of the following polymers is a heteropolymer?
Identify the polymer obtained by polymerization of n moles of acrylonitrile.
Which among the following monomers is used to prepare Teflon?
Which of the following monomers is used in the manufacture of Neoprene rubber?
Which among the following polymers is an example of addition polymer?
Which among the following polymers is used to manufacture chemical containers?
Which among the following is an example of addition polymer?
Which of the following polymers, need atleast one diene monomer for their preparation?
(i) Dacron
(ii) Buna-S
(iii) Neoprene
(iv) Novolac
Match the polymers given in Column I with their repeating units given in Column II.
| Column I | Column II |
| (i) Acrilan |
(a) \[\begin{array}{cc} |
| (ii) Polystyrene | (b) \[\begin{array}{cc} \ce{Cl}\phantom{.......}\\ |\phantom{........}\\ \phantom{}\ce{-(CH2 - C = CH - CH2)\underset{n}{-}} \end{array}\] |
| (iii) Neoprene | (c) \[\begin{array}{cc} \phantom{................................}\ce{CN}\\ \phantom{..............................}|\\ \ce{-(CH2 - CH = CH - CH2 - CH2 - CH)\underset{n}{-}} \end{array}\] |
| (iv) Novolac | (d) \[\begin{array}{cc} \ce{-(CH2 - CH)\underset{n}{-}}\\ \phantom{.....}|\\ \phantom{.......}\ce{CN} \end{array}\] |
| (v) Buna—N | (e) 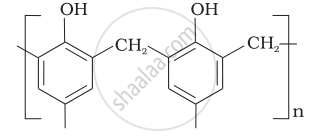 |
| (f) \[\begin{array}{cc} \ce{-(CH2 - CH)\underset{n}{-}}\\ \phantom{.....}|\\ \phantom{......}\ce{Cl} \end{array}\] |
Which of the following products is formed when benzaldehyde is treated with CH3MgBr and the addition product so obtained is subjected to acid hydrolysis?
The monomer of Teflon is ______.
Which of the following polymers is synthesized using a free radical polymerisation technique?
Nylon threads are made of ______.
The catalyst used for the polymerisation of olefins is ______.
Which among the following polymers has high tensile strength and is used to obtain tyre cords?
Which of the following is a polymer of enzyme?
Which of the foolowing polymer is used in the manufacture of insulators.
Answer the following.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
The monomer used in preparation of teflon is ______.
Write the structure and name of monomer of Nylon-6.
Write the structure and name of monomer of Natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
Name and draw structure of the repeating unit in natural rubber.
Write the structure of isoprene and the polymer obtained from it.
