Advertisements
Advertisements
प्रश्न
Write short notes on ortho, para directors in aromatic electrophilic substitution reactions.
उत्तर
- All the activating groups are ‘ortho-para’ directors.
Example: – OH, – NH2, -NHR, -NHCOCH3, -OCH3 -CH3 – C2H5 etc. - Let us consider the directive influences of phenolic (-OH) group. Phenol is the resonance hybrid of following structures.
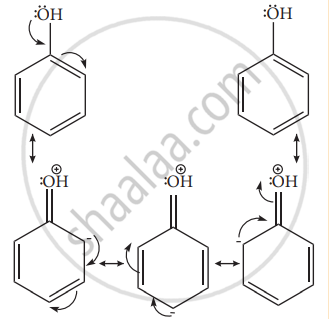
- In these resonance structures, the (-) charge residue is present on ortho and para position of ring structure. It is quite evident that the lone pair of electron on the atom which is attached to the ring involves in resonance and makes the ring more electron rich than benzene. The electron density at ortho and para positions increases as compared to the meta position. Therefore phenolic group activates the benzene ring for electrophilic attack at ‘ortho’ and ‘para positions and hence -OH group is an ortho-para director and activator.
- In aryl halides, the strong -I effect of the halogens (electron withdrawing tendency) decreases the electron density of benzene ring, thereby deactivating for electrophilic attack. However the presence of lone pair on halogens involved in the resonance with pi electrons of benzene ring, increases electron density at ortho and para position. Hence the halogen group is an ortho-para director and deactivator.
APPEARS IN
संबंधित प्रश्न
Name the following:
The hydrocarbon said to possess carcinogenic property.
Write the balanced chemical reaction to get benzene from Phenol.
Predict the possible product of the following reaction.
sulphonation of chlorobenzene
Identify giving reason whether the following compound is aromatic or not.

Identify giving reason whether the following compound is aromatic or not.
2 – butyne on chlorination gives ______.
How does Huckel rule help to decide the aromatic character of a compound?
Suggest the route for the preparation of the following from benzene.
4 – chlorotoluene
Suggest the route for the preparation of the following from benzene.
Bromo benzene
Identify the hydrocarbon compound from following containing carbon atoms in the range of C6 to C8?
