Advertisements
Advertisements
प्रश्न
Write the product formed when alkyl halide reacts with silver nitrite.
उत्तर
\[\ce{\underset{(Alkyl halide)}{R - X} + \underset{(Silver nitrite)}{Ag NO2} -> \underset{Nitroalkane}{R - NO2 + Ag - X}}\]
Nitroalkane is formed when alkyl halide reacts with silver nitrite.
APPEARS IN
संबंधित प्रश्न
Complete the following reaction giving major products.
\[\ce{CH3 - CH = CH2 ->[HBr][peroxide] A ->[alc. KOH] B}\]
Complete the following reaction giving major products.
\[\begin{array}{cc}\ce{CH3 - CH - CH3 ->[Red P/Br2] A ->[Ag2O/H2O]B}\\|\phantom{.........................}\\
\ce{OH\phantom{.......................}}\end{array}\]
Name the reagent used to bring about the following conversion.
Ethyl bromide to ethyl isocyanide
Arrange the following in the increasing order of boiling points.
- 1-Bromopropane
- 2- Bromopropane
- 1- Bromobutane
- 1-Bromo-2-methylpropane
Convert the following:
2-Chloropropane to propan-1-ol
HCl is added to a hydrocarbon ‘A’ \[\ce{(C4H8)}\] to give a compound ‘B’ which on hydrolysis with aqueous alkali forms tertiary alcohol ‘C’ \[\ce{(C4H10O)}\]. Identify ‘A’ ,‘B’ and ‘C’.
Write the correct order of increasing ease of dehydrohalogenation.
\[\ce{\underset{\text{(I)}}{CH3 - CH2 - CH2 - Cl}}\]
\[\ce{\underset{\text{(II)}}{CH3 - CH(Cl) - CH3}}\]
\[\begin{array}{cc}
\ce{CH3}\\
|\\
\ce{CH3-C-Cl}\phantom{...}\\
|\\
\ce{CH3}\\
\ce{(III)}
\end{array}\]
Explain primary benzylic halide shows higher reactivity by SN1 mechanism than other primary alkyl halide.
In alkaline hydrolysis of tertiary butyl bromide, ____________.
Which of the following is a primary halide?
Which of the following is least reactive towards SN1 reactions?
Ethyl bromide undergoes the following reaction:
\[\ce{\underset{Ethyl bromide}{C2H5Br} + \underset{(aq.)}{KOH} ->[\Delta] \underset{Ethyl alcohol}{C2H5OH} + KBr}\]
Which of the following is a WRONG statement?
The following mechanism has been proposed for the reaction of NO2 with F2 to form NO2F:
\[\ce{NO2_{(g)} + F2_{(g)} -> NO2F_{(g)} + F_{(g)} (slow)}\]
\[\ce{F_{(g)} + NO2_{(g)} -> NO2F_{(g)} (fast)}\]
The order of the reaction with respect to NO2(g)
How many metameric ethers are represented by the molecular formula C4H10O?
The SN2 reaction of a compound containing an asymmetric carbon atom always gives ____________.
Identify 'B' in the following reaction.
\[\ce{2-Bromobutane ->[KOH alc.][\Delta] A ->[HI] B}\]
The order of reactivities of the following alkyl halides for an SN2 reaction is ______.
The order of reactivity of the given haloalkanes towards nucleophiles is:
With which halogen the reactions, of alkanes are explosive?
The compound that reacts the fastest with sodium methoxide is ______.
Explain the dehydrohalogenation reaction of 2-chlorobutane.
Complete the following reaction giving a major product.
\[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
|\phantom{...................}\\
\ce{CH3 - C - CH2 - Cl->[Na/dry ether]A}\\
|\phantom{..................}\\
\ce{CH3}\phantom{................}
\end{array}\]
Observe the following and answer the question given below:
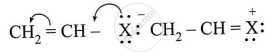
Comment on the bond length of C-X bond in it.
Observe the following and answer the question given below:
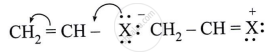
Can react by SN1 mechanism? Justify your answer.
Complete the following reaction sequences by writing the structural formulae of the organic compounds 'A', 'B' and 'C'.
\[\ce{2-Bromobutan ->[alc. KOH] A ->[][Br2] B ->[][NANH2] C}\]
Complete the following reaction giving major product.
\[\begin{array}{cc}
\ce{CH3}\phantom{...............}\\
|\phantom{..................}\\
\ce{CH3 - C - CH2 - Cl->[Na/dry ether]A}\\
|\phantom{..................}\\
\ce{CH3}\phantom{...............}\\
\end{array}\]
Complete the reaction:
\[\ce{CH3CH2Cl ->[AgCN][alc.\Delta]}\] ?
Complete the following reaction giving major product.
\[\begin{array}{cc}
\ce{CH3}\phantom{................}\\
|\phantom{...................}\\
\ce{CH3 - C - CH2 - Cl->[Na/ dry ether]A}\\
|\phantom{...................}\\
\ce{CH3}\phantom{................}\\
\end{array}\]
