Advertisements
Advertisements
Question
A gas is liberated immediately with a brisk effervescence, when you add acetic acid to sodium hydrogen carbonate powder in a test tube. Name the gas and describe the test that confirms the identity of the gas.
Solution
When acetic acid (CH3COOH) is added to the sodium hydrogen carbonate (NaHCO3) powder in a test tube, then the products obtained are sodium acetate, carbon dioxide and water.
Observations in this reaction are:
Brisk effervescence is seen because of CO2 gas escaping out from the reaction mixture. The gas turns lime water milky that confirms the identity of CO2 gas.
Chemical reactions involved:
CH3COOH + NaHCO3 →CH3COONa + CO2↑ + H2O
Ca(OH)2 + CO2 → CaCO3↓ + H2O
APPEARS IN
RELATED QUESTIONS
Consider the following comments about saponification reactions:
I. Heat is evolved in these reactions.
II. For quick precipitation of soap, sodium chloride is added to the reaction mixtures.
III. Saponification reactions are a special kind of neutralisation reactions.
IV. Soaps are basic salts of long-chain fatty acids.
The correct comments are
(a) I, II and III
(b) II, III and IV
(c) I, II and IV
(d) Only I and IV
How would you distinguish between ethanol and ethanoic acid by chemical test?
An organic compound X of molecular formula C2H4O2 gives brisk effervescence with sodium hydrogen carbonate. Give the name and formula of X.
When ethanoic acid reacts with sodium hydrogen carbonate, then a salt X is formed and a gas Y is evolved. Name the salt X and gas. Y Describe an activity with the help of a labelled diagram of the apparatus used to prove that the evolved gas is the one which you have named. Also write the chemical equation of the reaction involved.
Name the functional group present in the following compound:
C2H5CHO
What is vinegar and glacial acetic acid?
Write the characteristics of ethanoic acid.
The reaction between acetic acid and sodium carbonate is shown in the following figure.
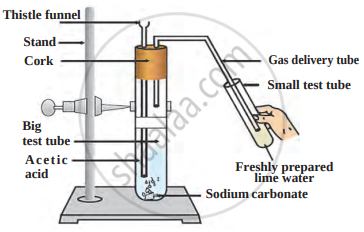
Answer the questions with the help of a diagram.
- Which gas does come out as effervescence in the big test tube?
- What is the colour change in the lime water present in the small test tube?
- Write the related reaction.
What happens when a small piece of sodium is dropped in ethanol? Write the equation for this reactions.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
