Advertisements
Advertisements
Question
How is ethanoic acid obtained from ethanol? Write down the chemical equation of the reaction involved.
Solution
When ethanol (ethyl alcohol) is heated in the presence of alkaline potassium permanganate solution or acidified potassium dichromate solution, it gets oxidised to ethanoic acid. This reaction is known as oxidation reaction.
The chemical equation for the above reaction is:
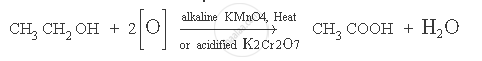
APPEARS IN
RELATED QUESTIONS
Name the following:
The distinctive reaction that takes place when ethanol is treated with acetic acid.
When zinc powder is added to acetic acid ______________
(a) the mixture becomes warm
(b) a gas is evolved
(c) the colour of the mixture becomes yellow
(d) a solid settles at the bottom
An organic compound A (molecular formula C2H4O2) reacts with Na metal to form a compound B and evolves a gas which burns with a pop sound. Compound A on treatment with an alcohol C in the presence of a little of concentrated sulphuric acid forms a sweet-smelling compound D (molecular formula C3H6O2). Compound D on treatment with NaOH solution gives back B and C. Identify A, B, C and
What do you notice when acetic acid reacts with alkalies?
A student is studying the properties of acetic acid in his school laboratory. List two physical and two chemical properties which he must observe and note in his record book.
What is vinegar and glacial acetic acid?
Give balanced chemical equations for the following conversion :
Ethanoic acid to ethyl ethanote
Ethanoic acid _________.
Write the molecular formula of the given compound.
Sodium ethanoate
A student while observing the properties of acetic acid would report that this smells like ______.
