Advertisements
Advertisements
Question
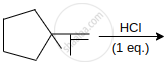
An alkyl halide C5H11Br (A) reacts with ethanolic KOH to give an alkene ‘B’, which reacts with Br2 to give a compound ‘C’, which on dehydrobromination gives an alkyne ‘D’. On treatment with sodium metal in liquid ammonia one mole of ‘D’ gives one mole of the sodium salt of ‘D’ and half a mole of hydrogen gas. Complete hydrogenation of ‘D’ yields a straight-chain alkane. Identify A, B, C and D. Give the reactions invovled.
Solution
\[\ce{\underset{(A)}{C5HBr} ->[Alc.KOH] \underset{(B)}{Alkene (C5H10)} ->[Br2 in CS2] \underset{(C)}{C5H10Br2} ->[Alc.KOH][-2HBr] \underset{D (Alkyne)}{C5H8} ->[Na-liq.NH3] \underset{Sodium alkylide}{C5H7 - Na + 1/2 H2}}\]
The reactions suggest that (D) is a terminal alkyne. This means triple bond is at the end of the chain. It could be either (l) or (II).
\[\ce{\underset{I}{CH3 - CH2 - CH2 - CH2 ≡ CH}}\]
\[\begin{array}{cc}
\ce{CH3 - CH - C ≡ CH}\\
|\phantom{......}\\
\ce{\underset{II}{CH3}}\phantom{...}
\end{array}\]
Since alkyne‘D’ on hydrogenation yields straight-chain alkane, therefore structure I is the structure of alkyne (D).
Hence, the structures of A, B and C are as follows:
(A) CH3 – CH2 – CH2 – CH2 – CHBr
(B) CH3 – CH2 – CH2 – CH = CH2
(C) CH3 – CH2 – CH2 – CH(Br) – CH2Br
APPEARS IN
RELATED QUESTIONS
Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate your answer by taking one example
Arrange the following alkyl halides in decreasing order of the rate of β– elimination reaction with alcoholic KOH.
| (A) | \[\begin{array}{cc} \ce{H}\phantom{...}\\ |\phantom{...}\\ \ce{CH3 - C - CH2Br}\\ |\phantom{...}\\ \ce{CH3}\phantom{} \end{array}\] |
| (B) | \[\ce{CH3 - CH2 - Br}\] |
| (C) | \[\ce{CH3 - CH2 - CH2 - Br}\] |
Which alcohol can be converted most easily to alkenes?
Arrange the following alkyl halides in decreasing order of the rate of ~ elimination reaction with alcoholic KOH:
- \[\begin{array}{cc}
\ce{H}\phantom{...}\\
|\phantom{...}\\
\phantom{}\ce{CH3 - C - CH2Br}\\
|\phantom{...}\\
\ce{CH3}\phantom{}
\end{array}\] - CH3 – CH2 – Br
- CH3 – CH2 – CH2 – Br
The major product formed in the dehydrohalogenation reaction of 2-Bromo pentane is Pent-2-ene. This product formation is based on the?
The major product in the following reaction is:
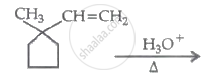
\[\ce{(A) ->[Cl2/hv] (B) ->[aq. KOH] (C) ->[O] CH3CHO}\]
Identify A, B and C:
What are A and B in the following reaction?
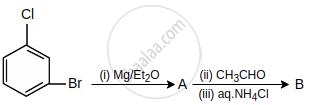
Major product of the reaction is: