Advertisements
Advertisements
Question
Assertion (A): Among isomeric pentanes, 2, 2-dimethylpentane has highest boiling point.
Reason (R): Branching does not affect the boiling point.
Options
Both A and R are correct and R is the correct explanation of A.
Both A and R are correct but R is not the correct explanation of A.
Both A and R are not correct.
A is not correct but R is correct.
Solution
Both A and R are not correct.
Explanation:
The boiling decreases with branching due to the decrease in surface area, which leads to a decrease in van der Waals force.
APPEARS IN
RELATED QUESTIONS
Write IUPAC name of the following compound:
CH2 = CH - C ≡ C - CH3
Write IUPAC name of the following compound:

Write IUPAC name of the following compound:
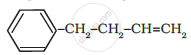
Write IUPAC name of the following compound:

Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3(CH2)4 CH(CH2)3 CH3}\\
|\phantom{..}\\
\phantom{..............}\ce{CH2 - CH(CH3)2}
\end{array}\]
Write IUPAC name of the following compound:
\[\begin{array}{cc}
\ce{CH3 - CH = CH - CH2 - CH = CH - CH - CH2 - CH = CH2}\\
\phantom{.................}|\\\phantom{.....................}\ce{C2H5}\end{array}\]
Arrange the following in decreasing order of their boiling points.
(A) n-butane
(B) 2-methylbutane
(C) n-pentane
(D) 2, 2-dimethylpropane
Match the reagent from Column I which on reaction with CH3 – CH = CH2 gives some product given in Column II as per the codes given below:
| Column I | Column II |
| (i) O3/Zn + H2O | (a) Acetic acid and CO2 |
| (ii) KMnO4/H+ | (b) Propan-1-ol |
| (iii) KMnO4/OH– | (c) Propan-2-ol |
| (iv) H2O/H+ | (d) Acetaldehyde and formaldehyde |
| (v) B2H6/NaOH and H2O2 | (e) Propane-1, 2-diol |
Two hydrocarbons such as octane and decane differ in molecular mass by ______ units.
Observed structures of the following compounds:
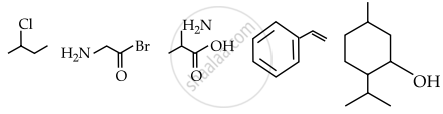
The total number of structures/compounds which possess asymmetric carbon atoms.
