Advertisements
Advertisements
Question
Assertion (A): Nitration of benzene with nitric acid requires the use of concentrated sulphuric acid.
Reason (R): The mixture of concentrated sulphuric acid and concentrated nitric acid produces the electrophile, \[\ce{NO^{+}2}\].
Options
Both A and R are correct and R is the correct explanation of A.
Both A and R are correct but R is not the correct explanation of A.
Both A and R are not correct.
A is not correct but R is correct.
Solution
Both A and R are correct and R is the correct explanation of A.
Explanation:
The sulphuric acid helps in furnishing \[\ce{NO^{+}2}\] (electrophile).

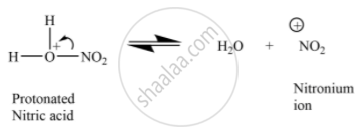
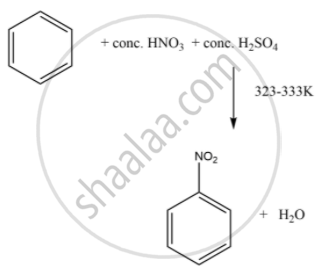
APPEARS IN
RELATED QUESTIONS
Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
For an electrophilic substitution reaction, the presence of a halogen atom in the benzene ring:
(i) deactivates the ring by inductive effect
(ii) deactivates the ring by resonance
(iii) increases the charge density at ortho and para position relative to meta position by resonance
(iv) directs the incoming electrophile to meta position by increasing the charge density relative to ortho and para position.
In an electrophilic substitution reaction of nitrobenzene, the presence of nitro group:
(i) Deactivates the ring by inductive effect.
(ii) Activates the ring by inductive effect.
(iii) Decreases the charge density at ortho and para position of the ring relative to meta position by resonance.
(iv) Increases the charge density at meta position relative to the ortho and para positions of the ring by resonance.
Why do alkenes prefer to undergo electrophilic addition reaction while arenes prefer electrophilic substitution reactions? Explain.
Why does presence of a nitro group make the benzene ring less reactive in comparison to the unsubstituted benzene ring. Explain.
Choose an INCORRECT statement about the electrophilic substitution reaction mechanism from the following:
In Friedel-Crafts alkylation of aniline, one gets ______.
Excess of isobutane on reactions with Br2 in presence of light at 125°C gives which one of the following, as the major product?
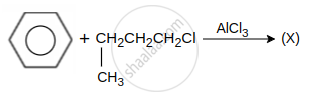
hydrocarbon (X) major product X is:
Benzene on nitration gives nitrobenzene in presence of HNO3 and H2SO4 mixture, where ______.
