Advertisements
Advertisements
Question
For an electrophilic substitution reaction, the presence of a halogen atom in the benzene ring:
(i) deactivates the ring by inductive effect
(ii) deactivates the ring by resonance
(iii) increases the charge density at ortho and para position relative to meta position by resonance
(iv) directs the incoming electrophile to meta position by increasing the charge density relative to ortho and para position.
Solution
(i) deactivates the ring by inductive effect
(iii) increases the charge density at ortho and para position relative to meta position by resonance
Explanation:
For an electrophilic substitution reaction, the presence of halogen atom in the benzene ring deactivates the ring by inductive effect and increases the charge density at ortho- and para-position relative to meta-position by resonance. When chlorine is attached to benzene ring, chlorine being more electronegative pulls the electrons because of its -1-effect. The electron cloud of benzene becomes less dense. Thus, chlorine makes the benzene ring in aryl halide somewhat deactivated. But due to resonance, the electron density on ortho- and para-positions is greater than on meta-position.
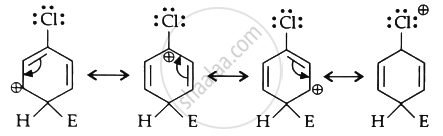
The last structure contributes more to the orientation and hence halogens are orthopara directing.
APPEARS IN
RELATED QUESTIONS
Arrange the set of compound in order of their decreasing relative reactivity with an electrophile, E+ Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene.
Arrange the set of compound in order of their decreasing relative reactivity with an electrophile, E+ Toluene, p-H3C–C6H4–NO2, p-O2N–C6H4–NO2.
Out of benzene, m–dinitrobenzene and toluene which will undergo nitration most easily and why?
Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
Why does presence of a nitro group make the benzene ring less reactive in comparison to the unsubstituted benzene ring. Explain.
Choose an INCORRECT statement about the electrophilic substitution reaction mechanism from the following:
In Friedel-Crafts alkylation of aniline, one gets ______.
Excess of isobutane on reactions with Br2 in presence of light at 125°C gives which one of the following, as the major product?
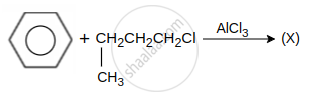
hydrocarbon (X) major product X is:
Which of the following would not give 2-phenylbutane as the major product in a Friedel-Crafts alkylation reaction?
