Advertisements
Advertisements
Question
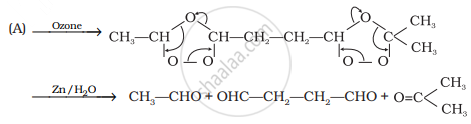
An unsaturated hydrocarbon ‘A’ adds two molecules of H2 and on reductive ozonolysis gives butane-1,4-dial, ethanal and propanone. Give the structure of ‘A’, write its IUPAC name and explain the reactions involved.
Solution
Two molecules of hydrogen add on ‘A’ this shows that ‘A’ is either an alkadiene or an alkyne.
On reductive ozonolysis ‘A’ gives three fragments, one of which is dialdehyde. Hence, the molecule has broken down at two sites. Therefore, ‘A’ has two double bonds. It gives the following three fragments:
OHC – CH2 – CH2 – CHO, CH3CHO and CH3 – CO – CH3
Hence, its structure as deduced from the three fragments must be
\[\begin{array}{cc}
\ce{CH3 - CH = CH - CH2 - CH2 - CH = C - CH3}\\
\phantom{.................................}|\\
\phantom{...................................}\ce{CH3}
\end{array}\]
(A)
Reactions
APPEARS IN
RELATED QUESTIONS
The increasing order of reduction of alkyl halides with zinc and dilute HCl is ______.
Write the structures and names of products obtained in the reactions of sodium with a mixture of 1-iodo-2-methylpropane and 2-iodopropane.
Identify a CORRECT statement about the preparation of alkanes from the following:
An alkyl halide by formation of its Grignard reagent and heating with water gives propane. What is the original alkyl halide?
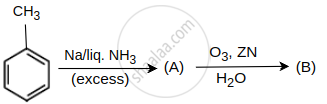
Identify products A and B.

In the given reaction,
\[\ce{2-Bromo-3, 3-dimethyl butane ->[C2H5OH][\underset{(Major product)}{'A'}]}\] Product A is ______.
The number of nitrogen atoms in a semicarbazone molecule of acetone is ______.
\[\ce{X + 3NH3 -> Y ->[H^+/H2O]}\]
H2N–CH2–COOH, compound X is:
\[\ce{X <-[red P][HI] CH3COOH ->[LiAlH4] Y.}\]
What does Not true for X and Y?
In the given reaction final product(s) will be: