Advertisements
Advertisements
Question
Answer the following.
What happens when Cl2 reacts with F2 in equal volume at 437 K.
Solution
When Cl2 reacts with F2 in equal volume at 437 K, chlorine monofluoride is formed.
\[\ce{\underset{\text{Chlorine}}{Cl2} + \underset{\text{Fluorine}}{F2} -> \underset{\text{Chlorine monofluoride}}{2ClF}}\]
APPEARS IN
RELATED QUESTIONS
Answer the following.
What is the oxidation state of ‘S’ in H2SO4?
Write the names and structural formulae of oxoacids of chlorine.
What is the oxidation state of sulfur in the following?
Sulfurous acid
Write three physical properties of sulfuric acid.
Write three uses of sulfuric acid.
Draw the structure of chloric acid.
Draw structure of chlorous acid.
The molecular formula of metaperiodic acid is ____________.
How many numbers of P—O—H and P—O—P bonds are present in pyrophosphoric acid respectively?
The following structure represents
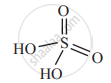
The molecular formula of peroxymonosulfuric acid is ____________.
The number of S = O bond in peroxy monosulfuric acid is _____________.
Which of the following oxyacid of sulphur contain S-O-S linkage?
Which of the following halogen does NOT form perhalic acid?
Which of the following structure represents chloric acid?
Identify the correct decreasing order of oxidizing power.
What is the oxidation state of chlorine atom in hypochlorous acid?
Which among the following halogens does not form the oxide of type OX2? (X = halogen)
How many lone pair of electrons are present on chlorine atoms in Hypochlorous acid?
Which among the following oxoacids of phosphorus shows a tendency of disproportionation?
Draw the structure of the following compound:
Peroxy disulphuric acid
Draw the structure of oxyacid of sulphur in which the oxidation state of sulphur is + 4.
How is chlorine obtained by oxidation of HCl. Write the reactions using two oxidizing agents.
Draw the structure of hypochlorous acid.
Draw the structure of peroxymonosulphuric acid.
