Advertisements
Advertisements
Question
Answer the following.
What happens when Br2 reacts with excess of F2.
Solution
When Br2 reacts with excess of F2, bromine trifluoride is formed.
\[\ce{\underset{\text{Bromine}}{Br2} + \underset{\text{Fluorine (Excess)}}{3F2} ->\underset{\text{Bromine trifluoride}}{2BrF3}}\]
APPEARS IN
RELATED QUESTIONS
Answer the following.
What is the oxidation state of ‘S’ in H2SO4?
Write the names and structural formulae of oxoacids of chlorine.
What is oxidation state of sulfur in the following?
Sulfuric acid
What is oxidation state of sulfur in the following?
Peroxymonosulfuric acid
Write three uses of sulfuric acid.
The formula of chlorous acid is ____________.
The molecular formula of metaperiodic acid is ____________.
The number of S - OH bond in thiosulfuric acid is ____________.
Which of the following molecular formula represents Marshall's acid?
The following structure represents
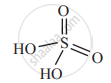
In which of the following oxoacids 'Cl' exhibit +5 oxidation state?
The number of S = O bond in peroxy monosulfuric acid is _____________.
Which of the following oxyacids of chlorine does not contain lone pair of electron on chlorine atom?
Which of the following oxyacid of sulphur contain S-O-S linkage?
Which of the following halogen does NOT form perhalic acid?
Which of the following structure represents chloric acid?
What is the oxidation state of chlorine atom in hypochlorous acid?
How many lone pairs of electrons are present on each oxygen atom in any oxyacids of chlorine?
Which among the following halogens does not form the oxide of type OX2? (X = halogen)
How many lone pair of electrons are present on chlorine atom in chlorus acid?
How many lone pair of electrons are present on chlorine atoms in Hypochlorous acid?
Draw the structure of oxyacid of sulphur in which the oxidation state of sulphur is + 4.
Draw structure of H2SO4.
Draw the structure of hypochlorous acid.
Draw the structure of peroxymonosulphuric acid.
