Advertisements
Advertisements
Question
Answer the following.
Why the elements belonging to the same group do have similar chemical properties?
Solution
- Chemical properties of elements depend upon their valency.
- Elements belonging to the same group have the same valency.
Hence, the elements belonging to the same group show similar chemical properties.
APPEARS IN
RELATED QUESTIONS
Explain the following.
The ionic radii of FΘ and Na⊕ are 133 and 98 pm, respectively.
Explain the following.
13Al is a metal, 14Si is a metalloid and 15P is a nonmetal.
Answer the following.
Ionization enthalpy of Li is 520 kJ mol-1 while that of F is 1681 kJ mol-1. Explain.
Answer the following.
Explain the screening effect with a suitable example.
Answer the following.
Why the second ionization enthalpy is greater than the first ionization enthalpy?
Answer the following.
Explain electronegativity and electron gain enthalpy. Which of the two can be measured experimentally?
Choose the correct option.
Consider the elements B, Al, Mg, and K predict the correct order of metallic character:
Choose the correct option.
Which of the following pairs is isoelectronic?
Answer the following question.
For the following pair, indicate which of the two species is of large size:
Mg2+ or Ca2+
Answer the following question.
Select the smaller ion form the following pair:
K+, Li+
With the help of a diagram answer the questions are given below:
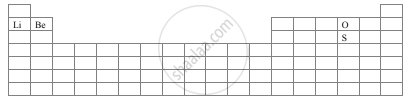
- Which atom should have smaller ionization energy, oxygen, or sulphur?
- The lithium forms +1 ions while beryllium forms +2 ions?
Define ionic radius
Define electronegativity
What are the valence electrons?
Define ionization enthalpy.
Name the factors on which ionization enthalpy depends?
How does ionization enthalpy vary down the group and across a period?
How the atomic size vary in a group and across a period? Explain with suitable example.
Give reason.
Alkali metals have low ionization enthalpies.
Give reason.
Inert gases have exceptionally high ionization enthalpies.
