Advertisements
Advertisements
Question
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution.
| (a) | 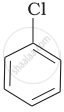 |
| (b) | 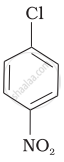 |
| (c) | 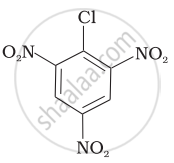 |
Options
(c) < (b) < (a)
(b) < (c) < (a)
(a) < (c) < (b)
(a) < (b) < (c)
Solution
(a) < (b) < (c)
Explanation:
The presence of an electron-withdrawing group (–NO2) at ortho- and para-positions increases the reactivity of haloarenes. The presence of a nitro group at ortho- and para-positions withdraws the electron density from the benzene ring and thus facilitates the attack of the nucleophile on haloarene. The carbanion thus formed is stabilised through resonance. The negative charge appeared at ortho- and para-positions with respect to the halogen substituent is stabilised by the –NO2 group.
APPEARS IN
RELATED QUESTIONS
What happens when \[\ce{CH3 - Br}\] is treated with KCN?
Write the final product(s) in each of the following reactions:
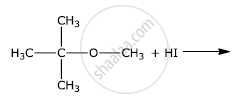
The presence of nitro group (−NO2) at o/p positions increases the reactivity of haloarenes towards nucleophilic substitution reactions.
How the following conversion can be carried out?
Chlorobenzene to p-nitrophenol
Write the product formed on reaction of D-glucose with Br2 water.
Write chemical equation in support of your answer.
Out of 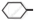 Cl and
Cl and 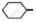 CH2- Cl, which one is more reactive towards nucleophilic substitution reaction and why?
CH2- Cl, which one is more reactive towards nucleophilic substitution reaction and why?
Which of the following compounds will give racemic mixture on nucleophilic substitution by \[\ce{OH-}\] ion?
(a) \[\begin{array}{cc}
\phantom{}\ce{CH3 - CH - Br}\\
\phantom{}|\\
\phantom{....}\ce{C2H5}\phantom{}
\end{array}\]
(b) \[\begin{array}{cc}
\phantom{..}\ce{Br}\\
\phantom{}|\\
\phantom{}\ce{CH3 - C - CH3}\\
\phantom{}|\\
\phantom{....}\ce{C2H5}\phantom{}
\end{array}\]
(c) \[\begin{array}{cc}
\phantom{....}\ce{CH3 - CH - CH2Br}\\
\phantom{}|\\
\phantom{....}\ce{C2H5}\phantom{}
\end{array}\]
Arrange the following compounds in increasing order of rate of reaction towards nucleophilic substitution.
| (a) | 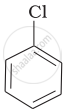 |
| (b) | 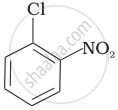 |
| (c) | 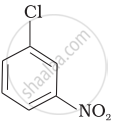 |
Haloarenes are less reactive than haloalkanes and haloalkenes. Explain.
Allyl chloride is hydrolysed more readily than n-propyl chloride. Why?
