Advertisements
Advertisements
Question
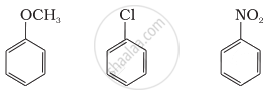
Arrange the following set of compounds in the order of their decreasing relative reactivity with an electrophile. Give reason.
Solution
The methoxy group (-OCH3) is electron releasing group. It increases the electron density in benzene nucleus due to resonance effect (+R-effect). Hence, it makes anisole more reactive than benzene towards the electrophile.

In case of alkyl halides, the electron density increases at ortho and para positions due to +R effect. However, the halogen atom also withdraws electrons from the ring because of its -I effect. Since the -I effect is stronger than the +R effect, the halogens are moderately deactivating. Thus, overall electron density on benzene ring decreases, which makes further substitution difficult. -NO2 group is electron-withdrawing group. It decreases the electron density in benzene nucleus due to its strong -R-effect and strong -I-effect. Hence, it makes nitrobenzene less reactive. Therefore, overall reactivity of these three compounds towards electrophiles decreases in the following order:
Anisole > Chlorobenzene > Nitrobenzene
In phenol, benzene ring has alternate single and double bonds while cyclohexanol is alicyclic compound.
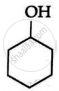
Cyclohexanol
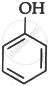
Phenol
APPEARS IN
RELATED QUESTIONS
Why is benzene extra ordinarily stable though it contains three double bonds?
Write down the products of ozonolysis of 1, 2-dimethylbenzene (o-xylene). How does the result support Kekulé structure for benzene?
Arrange the following carbanions in order of their decreasing stability.
(A) H3C – C ≡ C–
(B) H – C ≡ C–
(C) \[\ce{H3C - C\overset{-}{H2}}\]
Which of the following are correct?
(i) \[\ce{CH3 - O - CH^{⊕}2}\] is more stable than \[\ce{CH3 - CH^{⊕}2}\]
(ii) (CH3)2CH⊕ is less stable than \[\ce{CH3 - CH2 - CH^{⊕}2}\]
(iii) \[\ce{CH3 = CH2 - CH^{⊕}2}\] is more stable than \[\ce{CH3 - CH2 - CH^{⊕}2}\]
(iv) \[\ce{CH2 - CH^{⊕}}\] is more stable than \[\ce{CH3 - CH^{⊕}2}\]
How will you convert benzene into p – nitrobromobenzene
How will you convert benzene into m – nitrobromobenzene
The relative reactivity of 1°, 2°, 3° hydrogen’s towards chlorination is 1 : 3.8 : 5. Calculate the percentages of all monochlorinated products obtained from 2-methylbutane.
The number ratio of σ and π bonds in benzene is ______.
Mark the incorrect statement from the following:
