Advertisements
Advertisements
Question
‘Atoms of the same element or different from Dalton’s atomic theory by the Modern Atomic Theory.
Give two examples each of –
- Atoms of the same element
- Atoms of different elements combining to form a molecule.
Solution
Atoms of the same element or different elements combine to form a molecule.
Atoms of different elements forming a molecule
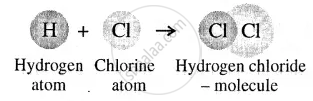
A molecule is the smallest particle of a pure substance – element or compound
- which can exist independently and
- retain the physical & chemical properties of the substance.
| Elements of same kind | Molecule of elements |
| Hydrogen atom |
Hydrogen molecule
|
| Nitrogen atom |
Nitrogen molecule
|
| Elements of same kind | Molecule of elements |
| Hydrogen [2 atoms] | 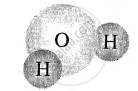 |
| Oxygen [1 atom] | water molecule |
APPEARS IN
RELATED QUESTIONS
What does 2H2S represent ?
Write the molecular formula of sulphuric acid.
Write the molecular formulae of Iron (II) sulphide :
Write the molecular formulae of Iron (III) sulphide:
Write the molecular formulae of Nitric acide :
The valency of sodium is one, write the molecular formula for sodium oxide ?
The valency of sodium is one, write the molecular formula for sodium sulphate ?
The valency of sodium is one, write the molecular formula for sodium carbonate ?
In hydrogen peroxide (H2O2), the proportion of hydrogen and oxygen by mass is :
If 1 gram of sulphur dioxide contains x molecules, how many molecules will be present in 1 gram of oxygen ?
(S = 32 u ; O = 16 u)
Which contains more molecules, 10 g of sulphur dioxide (SO2) or 10 g of oxygen (O2) ?
(Atomic masses : S = 32 u ; O = 16 u)
What is the number of electrons after the transfer of electrons in magnesium and sulphur atoms.
Diagrammatically represent the transfer of electrons.
Name the following:
A molecule of a compound containing two oxygen atoms and one nitrogen atom.
Molecule containing more than three atoms are known as ______.
Define molecule.
What is the fraction of the mass of water due to neutrons?
Express each of the following in kilograms
58.34 g
Express each of the following in kilograms
5.873×10−21g


