Advertisements
Advertisements
Question
Answer the following question.
Calculate the de-Broglie wavelength associated with the electron revolving in the first excited state of the hydrogen atom. The ground state energy of the hydrogen atom is – 13.6 eV.
Solution
de-Broglie wavelength `lambda = h/(mv) = h/p`, where p is momentum of electron
Kinetic energy (KE) and momentum (p) are related by, `"KE" = p^2/(2m) ("m"="mass")`
⇒ `p = sqrt(2m("KE")`
⇒ `lambda = h/sqrt(2m("KE")`
According to Bohr's model, `"Kinetic Energy of" e^- = |"Total Energy of " e^- |=|-(13.6xxZ^2)/(n^2)|e"V"`
for Hydrogen Z = 1 and first excited state implies n = 2
`"KE" = (13.6xx1^2)/(2^2) = 3.4 "eV"`
`= 3.4 xx 1.6 xx 10^-19 "J"`
`= 5.44 xx 10^-19 "J"`
putting the values in formula for wavelength we get,
`lambda = h/sqrt(2m("KE"))=(6.63xx10^-34)/sqrt(2xx9.1xx10^-31xx5.44xx10^-19)=6.66xx10^-10"m" = 6.66Å`
APPEARS IN
RELATED QUESTIONS
(i) State Bohr's quantization condition for defining stationary orbits. How does the de Broglie hypothesis explain the stationary orbits?
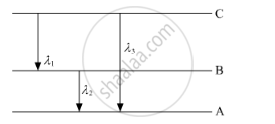
(ii) Find the relation between three wavelengths λ1, λ2 and λ3 from the energy-level diagram shown below.
State Bohr’s postulate of hydrogen atom which successfully explains the emission lines in the spectrum of hydrogen atom. Use Rydberg formula to determine the wavelength of Hα line. [Given: Rydberg constant R = 1.03 × 107 m−1]
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 × 107 ms–1, calculate the energy with which it is bound to the nucleus.
The electron in hydrogen atom is initially in the third excited state. What is the maximum number of spectral lines which can be emitted when it finally moves to the ground state?
The Bohr radius is given by `a_0 = (∈_0h^2)/{pime^2}`. Verify that the RHS has dimensions of length.
Which of the following is/are CORRECT according to Bohr's atomic theory?
(I) Energy is emitted when electron moves from a higher stationary state to a lower one.
(II) Orbits are arranged concentrically around the nucleus in an increasing order of energy.
(III) The energy of an electron in the orbit changes with time.
According to the Bohr theory of H-atom, the speed of the electron, its energy and the radius of its orbit varies with the principal quantum number n, respectively, as:
The number of times larger the spacing between the energy levels with n = 3 and n = 8 spacing between the energy level with n = 8 and n = 9 for the hydrogen atom is ______.
State three postulates of Bohr's theory of hydrogen atom.
The figure below is the Energy level diagram for the Hydrogen atom. Study the transitions shown and answer the following question:
- State the type of spectrum obtained.
- Name the series of spectrum obtained.

