Advertisements
Advertisements
Question
Calculate the work done in the following reaction at 50°C. State whether work is done on the system or by the system.
Solution
Δn = (moles of gaseous product) - (moles of gaseous reactant)
= 1-(1+
=
w = - ΔnRT
-(
=1342.7J
As work done is positive. Therefore work is done by surrounding on system.
(OR)
The standard enthalpy of combustion of formaldehyde Δ 0H0 = -571 kJ How much heat will be evolved in the formation of 22 g of CO2 ?
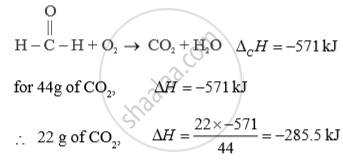
APPEARS IN
RELATED QUESTIONS
Define enthalpy of sublimation.
Calculate C-Cl bond enthalpy from following reaction:
CH3Cl(g) + Cl2(g) → Ch2Cl2(g) + HCl(g) ΔH° = -104KJ
If C-H, Cl-Cl and H-Cl bond enthalpies are 414, 243 and 431 KJ-Mol-1 respectively.
For the reaction: Cl2(g) → 2Cl(g), _______.
(A) ΔH is positive, ΔS is positive
(B) ΔH is positive, ΔS is negative
(C) ΔH is negative, ΔS is negative
(D) ΔH is negative, ΔS is positive
Calculate ΔH° for the reaction between ethene and water to form ethyl alcohol from the
following data:
ΔcH° C2H5OH(l) = -1368 kJ
ΔcH° C2H4(g) = -1410 kJ
Does the calculated ΔH° represent the enthalpy of formation of liquid ethanol?
Calculate the standard enthalpy of combustion of CH3COOH(l) from the following data:
Calculate the standard enthalpy of the reaction, 2C(graphite) + 3H2(g) → C2H6(g), ΔH° = ?
From the following ΔH° values
a)
b)
c) C(graphite) + O2(g) -> CO2(g). ΔH° = -393.5kJ
Calculate ∆H° for the following reaction:
2H3BO3(aq) → B2O3(s) + 3H2O(l)
a) H3BO3(aq) → HBO2(aq) + H2O(l) , ∆
b) H2B4O7(s) → 2B2O3(s) + H2O(l) , ∆
c)H2B4O7(s) + H2O(l) → 4HBO2(aq), ∆
