Advertisements
Advertisements
Question
Classify the following reaction into –
- Direct combination
- Decomposition
- Displacement
- Double decomposition
The reaction is – Molten zinc at high temperatures, burns in air to give zinc oxide.
Solution
Direct combination
\[\ce{2Zn + O2 -> 2ZnO}\]
APPEARS IN
RELATED QUESTIONS
Write the balanced chemical equation for the following and identify the type of reaction.
\[\ce{Hydrogen(g) + Chlorine(g) -> Hydrogen chloride (g)}\]
What type of chemical reaction take place when ammonia and hydrogen chloride are mixed?
What happens during a chemical reaction ?
Explain the terms with examples.
Combination reaction
Combustion reactions are always ____________.
The respiration process during which glucose undergoes slow combustion by combining with oxygen in the cells of our body to produce energy is a kind of:
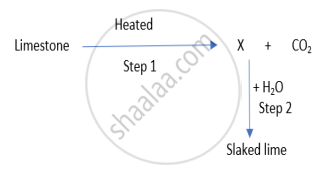
Identify the correct option from the given table which represents the type of reactions occurring in step 1 and step 2.
Identify the types of chemical reactions occurring during the combustion of fuel:
Balance the following chemical equation and identify the type of chemical reaction.
`"Mg"("s") + "Cl"_2("g") -> "MgCl"_2("s")`
Read the text below and answer the questions that follow:
A small amount of hydrochloric acid was taken in a test tube. The test tube was heated. A glass rod was dipped in the ammonia solution and held on the top of the test tube. A white smoke was seen emanating from the tip of the glass rod.
- What must have happened?
- Which colour of gas is formed?
- Write the chemical equation for the reaction.
