Advertisements
Advertisements
Question
Give a balanced equation for –
A direct combination reaction involving two elements, one of which is a non-metal
Solution
Balanced chemical equation: A combination reaction between calcium and oxygen (non-metal) to form calcium oxide.
\[\ce{2Ca + O2->2CaO}\]
APPEARS IN
RELATED QUESTIONS
Why is respiration considered an exothermic reaction? Explain.
One of the following is an endothermic reaction. This is:
(a) combination of carbon and oxygen to form carbon monoxide
(b) combination of nitrogen and oxygen to form nitrogen monoxide
(c) combination of glucose and oxygen to form carbon dioxide and water
(d) combination of zinc and hydrochloric acid to form zinc chloride and hydrogen
Define a chemical reaction.
Classify the following reaction as combination, decomposition, displacement, precipitation and neutralization. Also balance the equation.
\[\ce{CaCO3_{(s)} ->[heat]CaO_{(s)} + CO2_{(g)}}\]
What are chemical combination or synthesis reactions ? Chemical combination or synthesis:
Explain the terms with examples.
Combination reaction
Which of the following are exothermic processes?
(i) Reaction of water with quick lime
(ii) Dilution of an acid
(iii) Evaporation of water
(iv) Sublimation of camphor (crystals)
The respiration process during which glucose undergoes slow combustion by combining with oxygen in the cells of our body to produce energy is a kind of:
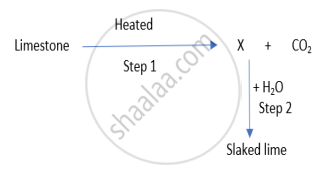
Identify the correct option from the given table which represents the type of reactions occurring in step 1 and step 2.
Balance the following chemical equation and identify the type of chemical reaction.
`"CaO"("s") + "SiO"_2("s") -> "CaSio"_3("s")`
