Advertisements
Advertisements
Question
Compare the chemistry of actinoids with that of the lanthanoids with special reference to chemical reactivity.
Solution 1
Generally, the initial members of the series are very reactive like calcium in their chemical behaviour, but with increasing atomic number they behave like aluminium.
The value of EΘ for the half-reaction \[\ce{Ln^{3+}_{ (aq)} + 3e- -> Ln_{(s)}}\] is in the range of −2.2 V to −2.4 V. For Eu, the value of EΘ is −2.0 V. Of course, there is a slight variation in the value. These metals combine with hydrogen on being gently heated in an atmosphere of hydrogen gas. On heating these metals with carbon, the carbides Ln3C, Ln2C3 and LnC2 are formed. These metals liberate hydrogen gas from dilute acids and on burning in an atmosphere of halogens, form halides. These form oxide M2O3 and hydroxide M(OH)3. Hydroxides are definite compounds and not just hydrated oxides. They are basic, like the oxides and hydroxides of alkaline earth metals. Their general reactions are shown in the figure.
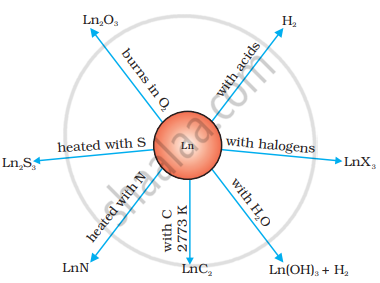
Actinoids are very reactive metals, especially when they are finely divided. The action of boiling water on them gives a mixture of oxide and hydride and combines with most non-metals at normal temperatures. Hydrochloric acid affects all metals, but most metals are little affected by nitric acid. The reason for this is that a protective surface of oxide is formed on these metals. Alkalis have no effect on these metals.
Solution 2
Actinoids are far more reactive than lanthanoids. They interact with nonmetals at moderate temperatures. In contrast, lanthanoids react at high temperatures. The majority of actinoids are attacked by HNO3 has a small effect on HCl, although the creation of a protective layer of oxide. Alkalies cause no reaction. Lanthanoids extract hydrogen from dilute. Acids and halogens burn together to generate halides.
Notes
Students should refer to the answer according to their questions.
APPEARS IN
RELATED QUESTIONS
Account for the following :
Zr and Hf have almost similar atomic radii.
Compare the chemistry of actinoids with that of lanthanoids with special reference to electronic configuration.
Write the different oxidation states of iron
Explain the cause of Lanthanoids contraction.
Answer the followiiig questions:
Which trivalent ion has maximum size in the Lanthanoid series i.e. Lanthanum ion (La3+) to Luteium ion (Lu3+)?
(at. no. of Lanthanum = 57 and Lutetium = 71)
What is lanthanoid contraction? Write the.............
Gadolinium belongs to 4f series. It’s atomic number is 64. Which of the following is the correct electronic configuration of gadolinium?
Although +3 is the characteristic oxidation state for lanthanoids but cerium also shows +4 oxidation state because:
(i) it has variable ionisation enthalpy
(ii) it has a tendency to attain noble gas configuration
(iii) it has a tendency to attain f 0 configuration
(iv) it resembles Pb4+
On the basis of Lanthanoid contraction, explain the following:
Nature of bonding in \[\ce{La2O3}\] and \[\ce{Lu2O3}\] .
On the basis of Lanthanoid contraction, explain the following:
Trends in the stability of oxo salts of lanthanoids from \[\ce{La}\] to \[\ce{Lu}\].
On the basis of Lanthanoid contraction, explain the following:
Trends in acidic character of lanthanoid oxides.
The titanium (Z = 22) compound that does not exist is:-
The lathanide ion that would show colour is ______.
Mischmetal is an alloy consisting mainly of ______.
Cerium (Z = S8) is an important member of lanthanoids. Which of the following statements about cerium is incorrect?
Write a note on lanthanoids.
Why is Mn2+ ion more stable than Fe2+ ion?
(Atomic numbers of Mn = 25 and Fe = 26)
Trivalent Lanthanoid ions such as La3+ (Z = 57) and Lu3+ (Z = 71) do not show any colour in their solution. Give a reason.
Mention alloy uses.
