Advertisements
Advertisements
Question
Complete the following reaction.
\[\ce{C6H5 - OH ->[Zn dust] A ->[CH3Cl][Anhydrous AlCl3] B ->[acid KMnO4] C}\]
Solution
\[\ce{\underset{(Ethanol)}{C6H5 - OH} ->[Zn dust][\Delta] \underset{\underset{(A)}{(Benzene)}}{C6H6} ->[CH3Cl][Anhydrous AlCl3] \underset{\underset{(B)}{(Toluene)}}{C6H5CH3} ->[KMnO4/H^+] \underset{\underset{(C)}{(Benzoic acid)}}{C6H5COOH}}\]
APPEARS IN
RELATED QUESTIONS
On reacting with neutral ferric chloride, phenol gives ____________.
Arrange the following in the increasing order of their boiling point and give a reason for your ordering.
Butan-2-ol, Butan-1-ol, 2-methyl propane-2-ol
What happens when 1-phenyl ethanol is treated with acidified KMnO4.
Write the structure of the aldehyde, carboxylic acid and ester that yield 4-methylpent-2-en-1-ol.
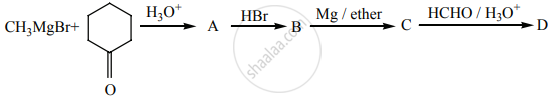
Identify A, B, C, D and write the complete equation.
Draw the major product formed when 1-ethoxyprop-1-ene is heated with one equivalent of HI
Predict the major product, when 2-methyl but -2-ene is converted into an alcohol in the following method.
Acid catalysed hydration
Identify the product(s) is/are formed when 1-methoxy propane is heated with excess HI. Name the mechanism involved in the reaction.
What will be the product (X and A)for the following reaction
\[\ce{acetylchloride ->[i) CH3MgBr][ii) H3O^+]X ->[acid K2Cr2O7]A}\]
Predict the major product, when 2-methyl but-2-ene is converted into an alcohol of the following method:
Acid catalysed hydration
